RITE AID ZINC COLD THERAPY- zincum gluconicum tablet
RITE AID CORPORATION
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
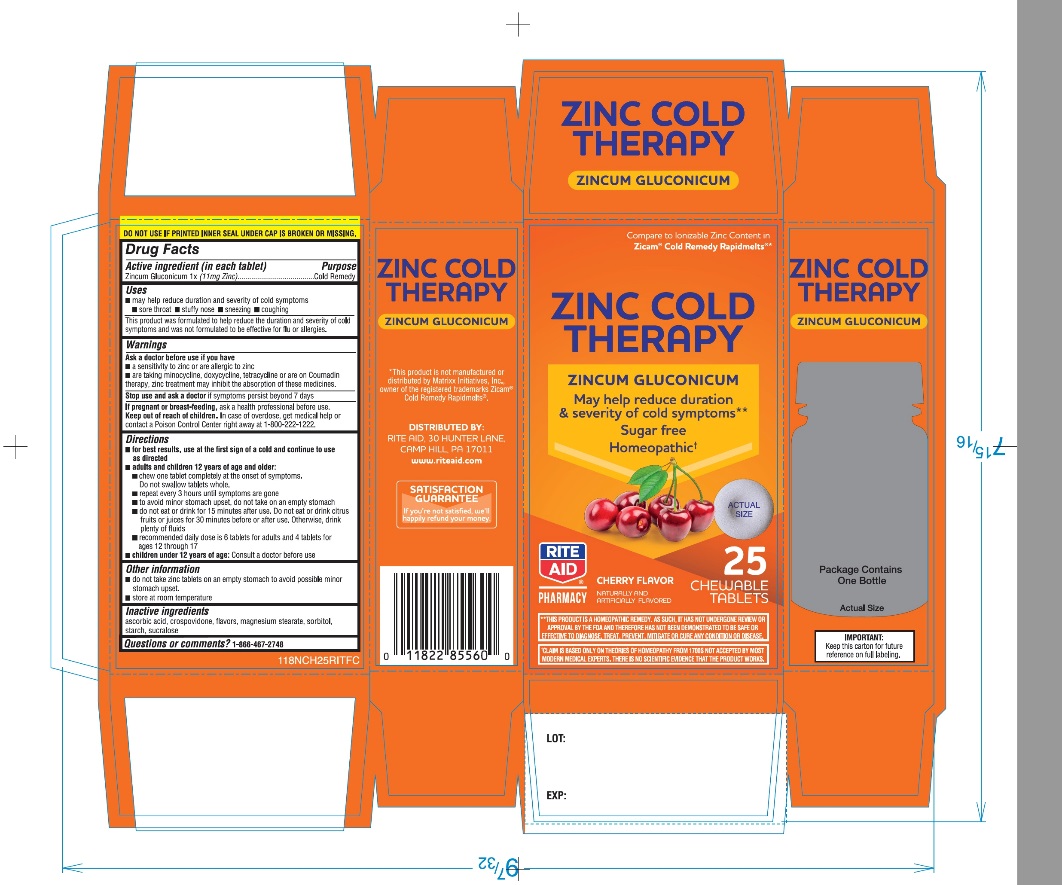
Rite Aid Zinc Cold Therapy Chewable Tablets
Uses
- ▪
- may help reduce duration of cold symptoms:
- ▪
- sore throat
- ▪
- stuffy nose
- ▪
- sneezing
- ▪
- coughing
- This product was formulated to help reduce the duration and severity of cold symptoms and was not formulated to be effective for flu or allergies.
Directions
- ▪
- for best results, use at the first sign of a cold and continue to use as directed
- ▪
- adults and children 12 years of age and older:
- ▪
- chew one tablet completely at the onset of symptoms. Do not swallow tablets whole.
- ▪
- repeat every 3 hours until symptoms are gone
- ▪
- to avoid minor stomach upset, do not take on an empty stomach.
- ▪
- do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- ▪
- recommended daily dose is 6 tablets for adults and 4 tablets for ages 12 through 17
- ▪
- Children under 12 years of age: Consult a doctor before use.
Other information
- ▪
- do not take zinc tablets on an empty stomach to avoid possible minor stomach upset.
- ▪
- store at room temperature
Inactive ingredients
ascorbic acid, crospovidone, flavors, magnesium stearate, sorbitol, starch ,sucralose.
PRINCIPAL DISPLAY PANEL
NDC 11822-1183-5
*Compare toIonizable Zinc Content in Zicam® Cold Remedy Rapid Melts®
Zinc Cold Therapy
ZINCUM GLUCONICUM
May help reduce the duration of cold symptoms**
Sugar Free
Homeopathic†
Cherry Flavor
Naturally and Artificially Flavored
25 CHEWABLE TABLETS
Package Contains One Bottle
DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING.
|
†CLAIM IS BASED ONLY ON THEORIES OF HOMEOPATHY FROM 1700S NOT ACCEPTED BY MOST MODERN MEDICAL EXPERTS .THERE IS NO SCIENTIFIC EVIDENCE THAT THE PRODUCT WORKS |
|
**THIS PRODUCT IS A HOMEOPATHIC REMEDY, AS SUCH, IT HAS NOT UNDERGONE REVIEW ON APPROVAL BY THE FDA AND THEREFORE HAS NOT BEEN DEMONSTRATED TO BE SAFE OR EFFECTIVE TO DIAGNOSE, TREAT, PREVENT, MITIGATE OR CURE ANY CONDITION OR DISEASE. |
|
IMPORTANT: Keep this carton for future reference on full labeling. |
*This product is not manufactured or distributed by Matrixx Initiatives, Inc., owner of the registered trademarks Zicam® Cold Remedy Rapidmelts®
Distributed by:
| RITE AID ZINC COLD THERAPY
zincum gluconicum tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - RITE AID CORPORATION (014578892) |