NORTHSIDE HOSPITAL AMENITY KIT--GI DEPT- alcohol
ASP Global, LLc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Northside Hospital Amenity Kit--GI Dept
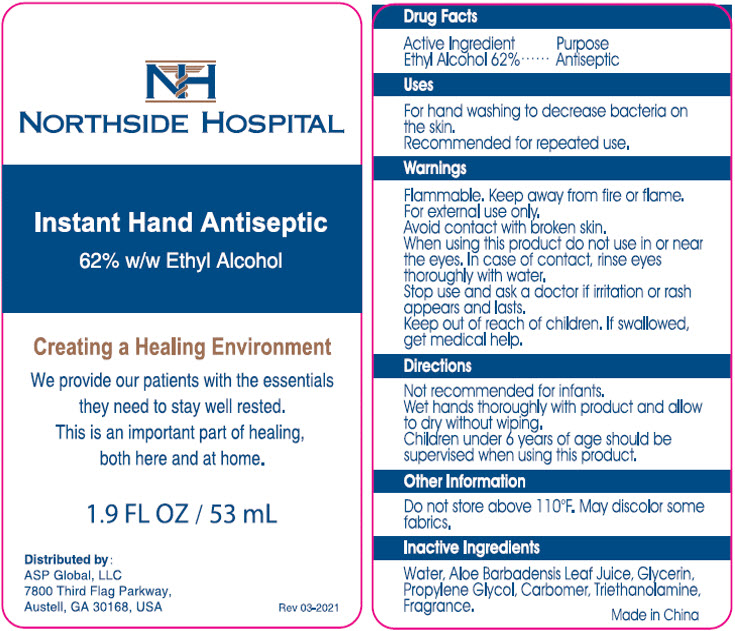
Warnings
Flammable. Keep away from fire or flame.
For external use only.
Avoid contact with broken skin.
Directions
Not recommended for infants.
Wet hands thoroughly with product and allow to dry without wiping.
Children under 6 years of age should be supervised when using this product.
Inactive Ingredients
Water, Aloe Barbadensis Leaf Juice, Glycerin, Propylene Glycol, Carbomer, Triethanolamine, Fragrance.
PRINCIPAL DISPLAY PANEL - 53 mL Bottle Label
NORTHSIDE HOSPITAL
Instant Hand Antiseptic
62% w/w Ethyl Alcohol
Creating a Healing Environment
We provide our patients with the essentials
they need to stay well rested.
This is an important part of healing,
both here and at home.
1.9 FL OZ / 53 mL
Distributed by:
ASP Global, LLC
7800 Third Flag Parkway,
Austell, GA 30168, USA
Rev 03-2021
PRINCIPAL DISPLAY PANEL - Kit Label
NORTHSIDE HOSPITAL
Item #: NSGIKIT01
Description: Amenity Kit, GI Dept
PO #:
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Qty: 30 KIT/CS
Carton #: X of XX
Net Wt.: XX KG
Gross Wt.: XX KG
Cubic Dimensions: NN x NN x NN CM
Made in China
Components Made in China:
- Hand Sanitizer
- Lip Balm
- Pen
- Sudoku book
- Questions for My Care Team Book
- Kit Case
NORTHSIDE HOSPITAL
Creating a Healing Environment
We provide our patients with the essentials
they need to stay well rested.
This is an important part of healing,
both here and at home.
Contents/Origins:
0.15 oz. net wt. Lip Balm,
1.9 fl. oz. Hand Sanitizer,
Pen, Puzzle Book, Question for My Care Team Book,
and Kit Case: Made in China
Lot #:
Exp:
Distributed by:
ASP Global, LLC
7800 Third Flag Parkway, Austell, GA 30168, USA
REV 06
NORTHSIDE HOSPITAL

| NORTHSIDE HOSPITAL AMENITY KIT--GI DEPT
alcohol kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - ASP Global, LLc (080361159) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shengzhou Kingbird Travel Products Co., Ltd. | 560219293 | PACK(59448-201) , LABEL(59448-201) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Colgate-Palmolive (Thailand) LTD | 672044552 | MANUFACTURE(59448-201) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nantong Health & Beyond Hygienic Products Inc. | 421280161 | MANUFACTURE(59448-201) | |