ZEJULA- niraparib capsule
GlaxoSmithKline LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZEJULA safely and effectively. See full prescribing information for ZEJULA.
ZEJULA (niraparib) capsules, for oral use Initial U.S. Approval: 2017 RECENT MAJOR CHANGESINDICATIONS AND USAGEZEJULA is a poly(ADP-ribose) polymerase (PARP) inhibitor indicated:
DOSAGE AND ADMINISTRATIONFirst-line maintenance treatment of advanced ovarian cancer:
For other indications: DOSAGE FORMS AND STRENGTHSCapsules: 100 mg (3) CONTRAINDICATIONSNone. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥10%) of patients who received ZEJULA were nausea, thrombocytopenia, anemia, fatigue, constipation, musculoskeletal pain, abdominal pain, vomiting, neutropenia, decreased appetite, leukopenia, insomnia, headache, dyspnea, rash, diarrhea, hypertension, cough, dizziness, acute kidney injury, urinary tract infection, and hypomagnesemia. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 4/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 First-Line Maintenance Treatment of Advanced Ovarian Cancer
ZEJULA is indicated for the maintenance treatment of adult patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to first-line platinum-based chemotherapy.
1.2 Maintenance Treatment of Recurrent Ovarian Cancer
ZEJULA is indicated for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy.
1.3 Treatment of Advanced Ovarian Cancer after Three or More Chemotherapies
ZEJULA is indicated for the treatment of adult patients with advanced ovarian, fallopian tube, or primary peritoneal cancer who have been treated with three or more prior chemotherapy regimens and whose cancer is associated with homologous recombination deficiency (HRD) positive status defined by either:
- •
- a deleterious or suspected deleterious BRCA mutation, or
- •
- genomic instability and who have progressed more than six months after response to the last platinum-based chemotherapy [see Clinical Studies (14.3)].
Select patients for therapy based on an FDA-approved companion diagnostic for ZEJULA.
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection for Treatment of Advanced Ovarian Cancer after Three or More Chemotherapies
Select patients for treatment of advanced ovarian cancer after three or more chemotherapy regimens associated with HRD positive status based on either deleterious or suspected deleterious BRCA mutation and/or genomic instability score (GIS) [see Clinical Studies (14.3)].
Information on FDA-approved tests for the detection of either deleterious or suspected deleterious BRCA mutation or genomic instability for this indication is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
Continue ZEJULA treatment until disease progression or unacceptable toxicity.
Instruct patients to take their dose of ZEJULA at approximately the same time each day. Advise patients to swallow each capsule whole and not to chew, crush, or split ZEJULA prior to swallowing. ZEJULA may be taken with or without food. Bedtime administration may be a potential method for managing nausea.
In the case of a missed dose of ZEJULA, instruct patients to take their next dose at its regularly scheduled time. If a patient vomits or misses a dose of ZEJULA, an additional dose should not be taken.
First-Line Maintenance Treatment of Advanced Ovarian Cancer
- •
- For patients weighing less than 77 kg (170 lbs) OR with a platelet count of less than 150,000/μL, the recommended dose is 200 mg (two 100-mg capsules) taken orally once daily.
- •
- For patients weighing greater than or equal to 77 kg (170 lbs) AND who have a platelet count greater than or equal to 150,000/μL, the recommended dose is 300 mg (three 100-mg capsules) taken orally once daily.
For the maintenance treatment of advanced ovarian cancer, patients should start treatment with ZEJULA no later than 12 weeks after their most recent platinum-containing regimen.
Maintenance Treatment of Recurrent Ovarian Cancer
The recommended dose of ZEJULA is 300 mg (three 100-mg capsules) taken orally once daily.
For the maintenance treatment of recurrent ovarian cancer, patients should start treatment with ZEJULA no later than 8 weeks after their most recent platinum-containing regimen.
Treatment of Advanced Ovarian Cancer after Three or More Chemotherapies
The recommended dose of ZEJULA is 300 mg (three 100-mg capsules) taken orally once daily.
2.3 Dosage Adjustments for Adverse Reactions
To manage adverse reactions, consider interruption of treatment, dose reduction, or dose discontinuation. The recommended dose modifications for adverse reactions are listed in Tables 1, 2 and 3.
| Starting dose level | 200 mg | 300 mg |
|---|---|---|
| a If further dose reduction below 100 mg/day is required, discontinue ZEJULA. | ||
|
First dose reduction |
100 mg/daya (one 100-mg capsule) |
200 mg/day (two 100-mg capsules) |
|
Second dose reduction |
Discontinue medication. |
100 mg/daya (one 100-mg capsule) |
| a CTCAE=Common Terminology Criteria for Adverse Events | ||||
|
Non-hematologic CTCAEa ≥ Grade 3 adverse reaction where prophylaxis is not considered feasible or adverse reaction persists despite treatment |
|
|||
|
CTCAE ≥ Grade 3 treatment-related adverse reaction lasting more than 28 days while patient is administered ZEJULA 100 mg/day |
Discontinue medication. |
|||
| a If myelodysplastic syndrome or acute myeloid leukemia (MDS/AML) is confirmed, discontinue ZEJULA [see Warnings and Precautions (5.1, 5.2)]. | ||||||
|
Monitor complete blood counts weekly for the first month, monthly for the next 11 months of treatment and periodically after this time [see Warnings and Precautions (5.1)]. |
||||||
|
Platelet count <100,000/µL |
First occurrence:
|
|||||
|
Second occurrence:
|
||||||
|
Neutrophil <1,000/µL or Hemoglobin <8 g/dL |
|
|||||
|
Hematologic adverse reaction requiring transfusion |
|
|||||
3 DOSAGE FORMS AND STRENGTHS
100 mg capsule having a white body with “100 mg” printed in black ink, and a purple cap with “Niraparib” printed in white ink.
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML), including cases with fatal outcome, have been reported in patients who received ZEJULA monotherapy in clinical trials. In 1785 patients treated with ZEJULA in clinical trials, MDS/AML occurred in 15 patients (0.8%).
The duration of therapy with ZEJULA in patients who developed secondary MDS/cancer therapy-related AML varied from 0.5 months to 4.9 years. All of these patients had received previous chemotherapy with platinum agents and/or other DNA-damaging agents including radiotherapy. Discontinue ZEJULA if MDS/AML is confirmed.
5.2 Bone Marrow Suppression
Hematologic adverse reactions (thrombocytopenia, anemia and neutropenia) have been reported in patients treated with ZEJULA.
In PRIMA, the overall incidence of Grade ≥3 thrombocytopenia, anemia and neutropenia were reported, respectively, in 39%, 31%, and 21% of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred, respectively, in 4%, 2%, and 2% of patients. In patients who were administered a starting dose of ZEJULA based on baseline weight or platelet count, Grade ≥3 thrombocytopenia, anemia and neutropenia were reported, respectively, in 22%, 23%, and 15% of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred, respectively, in 3%, 3%, and 2% of patients.
In NOVA, Grade ≥3 thrombocytopenia, anemia and neutropenia were reported, respectively, in 29%, 25%, and 20% of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred, respectively, in 3%, 1%, and 2% of patients.
In QUADRA, Grade ≥3 thrombocytopenia, anemia and neutropenia were reported, respectively, in 28%, 27%, and 13% of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred, respectively, in 4%, 2%, and 1% of patients.
Do not start ZEJULA until patients have recovered from hematological toxicity caused by previous chemotherapy (≤ Grade 1). Monitor complete blood counts weekly for the first month, monthly for the next 11 months of treatment, and periodically after this time. If hematological toxicities do not resolve within 28 days following interruption, discontinue ZEJULA, and refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics [see Dosage and Administration (2.3)].
5.3 Cardiovascular Effects
Hypertension and hypertensive crisis have been reported in patients treated with ZEJULA.
In PRIMA, Grade 3-4 hypertension occurred in 6% of ZEJULA-treated patients compared to 1% of placebo-treated patients with a median time from first dose to first onset of 43 days (range: 1 to 531 days) and with a median duration of 12 days (range: 1 to 61 days). There were no discontinuations due to hypertension.
In NOVA, Grade 3-4 hypertension occurred in 9% of ZEJULA-treated patients compared to 2% of placebo-treated patients with a median time from first dose to first onset of 77 days (range: 4 to 504 days) and with a median duration of 15 days (range: 1 to 86 days). Discontinuation due to hypertension occurred in <1% of patients.
In QUADRA, Grade 3-4 hypertension occurred in 5% of ZEJULA-treated patients with a median time from first dose to first onset of 15 days (range: 1 to 316 days) and with a median duration of 7 days (range: 1 to 118 days). Discontinuation due to hypertension occurred in <0.2% of patients.
Monitor blood pressure and heart rate at least weekly for the first two months, then monthly for the first year and periodically thereafter during treatment with ZEJULA. Closely monitor patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. Medically manage hypertension with antihypertensive medications and adjustment of the ZEJULA dose, if necessary [see Dosage and Administration (2.3) and Nonclinical Toxicology (13.2)].
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, ZEJULA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. ZEJULA has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow) [see Warnings and Precautions (5.2) and Nonclinical Toxicology (13.1)]. Due to the potential risk to a fetus based on its mechanism of action, animal developmental and reproductive toxicology studies were not conducted with niraparib.
Apprise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after the last dose of ZEJULA [see Use in Specific Populations (8.1, 8.3)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Myelodysplastic Syndrome/Acute Myeloid Leukemia [see Warnings and Precautions (5.1)]
- •
- Bone Marrow Suppression [see Warnings and Precautions (5.2)]
- •
- Cardiovascular Effects [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions of all grades in >10% of 1314 patients who received ZEJULA in the pooled PRIMA, NOVA and QUADRA trials were nausea (65%), thrombocytopenia (60%), anemia (56%), fatigue (55%), constipation (39%), musculoskeletal pain (36%), abdominal pain (35%), vomiting (33%), neutropenia (31%), decreased appetite (24%), leukopenia (24%), insomnia (23%), headache (23%), dyspnea (22%), rash (21%), diarrhea (18%), hypertension (17%), cough (16%), dizziness (14%), acute kidney injury (13%), urinary tract infection (12%), and hypomagnesemia (11%).
First-Line Maintenance Treatment of Advanced Ovarian Cancer
The safety of ZEJULA for the treatment of patients with advanced ovarian cancer following first-line treatment with platinum-based chemotherapy was studied in the PRIMA trial, a placebo-controlled, double-blind study in which 728 patients received niraparib or placebo. Among patients who received ZEJULA, the median duration of treatment was 11.1 months (range: 0.03 to 29 months).
All Patients Receiving ZEJULA in PRIMA
Serious adverse reactions occurred in 32% of patients receiving ZEJULA. Serious adverse reactions in >2% of patients were thrombocytopenia (16%), anemia (6%), and small intestinal obstruction (2.9%). Fatal adverse reactions occurred in 0.4% of patients, including intestinal perforation and pleural effusion (one patient each).
Permanent discontinuation due to adverse reactions occurred in 12% of patients who received ZEJULA. Adverse reactions resulting in permanent discontinuation in >1% of patients who received ZEJULA included thrombocytopenia (3.7%), anemia (1.9%), nausea and neutropenia (1.2% each).
Adverse reactions led to dose reduction or interruption in 80% of patients, most frequently from thrombocytopenia (56%), anemia (33%), and neutropenia (20%).
Table 4 and Table 5 summarize the common adverse reactions and abnormal laboratory findings, respectively, observed in all patients treated with ZEJULA in the PRIMA study.
| Grades 1-4b | Grades 3-4b | |||
|---|---|---|---|---|
| ZEJULA
N=484 % | Placebo
N=244 % | ZEJULA
N=484 % | Placebo
N=244 % |
|
|
Blood and Lymphatic System Disorders |
||||
|
Thrombocytopenia |
66 |
5 |
39 |
0.4 |
|
Anemia |
64 |
18 |
31 |
2 |
|
Neutropeniac |
42 |
8 |
21 |
1 |
|
Leukopeniad |
28 |
9 |
5 |
0.4 |
|
Gastrointestinal Disorders |
||||
|
Nausea |
57 |
28 |
1 |
1 |
|
Constipation |
40 |
20 |
1 |
0.4 |
|
Vomiting |
22 |
12 |
1 |
1 |
|
General Disorders and Administration Site Conditions |
||||
|
Fatigue |
51 |
41 |
3 |
1 |
|
Investigations |
||||
|
AST/ALT elevation |
14 |
7 |
3 |
0.8 |
|
Metabolism and Nutrition Disorders |
||||
|
Decreased appetite |
19 |
8 |
1 |
0 |
|
Musculoskeletal and Connective Tissue Disorders |
||||
|
Musculoskeletal pain |
39 |
38 |
1 |
0 |
|
Nervous System Disorders |
||||
|
Headache |
26 |
15 |
0.4 |
0 |
|
Dizziness |
19 |
13 |
0 |
0.4 |
|
Psychiatric Disorders |
||||
|
Insomnia |
25 |
15 |
1 |
0.4 |
|
Renal and Urinary Disorders |
||||
|
Acute kidney injurye |
12 |
5 |
0.2 |
0 |
|
Respiratory, Thoracic and Mediastinal Disorders |
||||
|
Dyspnea |
22 |
13 |
0.4 |
1 |
|
Cough |
18 |
15 |
0 |
0.4 |
|
Vascular Disorders |
||||
|
Hypertension |
18 |
7 |
6 |
1 |
aAll adverse reactions in the table consist of grouped preferred terms except for nausea, vomiting, decreased appetite, headache and insomnia, which are single preferred terms.
bCTCAE=Common Terminology Criteria for Adverse Events version 4.02
cincludes neutropenia, neutropenic infection, neutropenic sepsis, febrile neutropenia.
dincludes leukopenia, lymphocyte count decreased, lymphopenia, white blood cell count decreased.
eincludes blood creatinine increased, blood urea increased, acute kidney injury, renal failure, blood creatine increased.
|
Grades 1-4 |
Grades 3-4 |
|||
|
ZEJULA
N=484
|
Placebo
N=244
|
ZEJULA
N=484
|
Placebo
N=244
|
|
|
Decreased hemoglobin |
87 |
66 |
29 |
1 |
|
Decreased platelets |
74 |
13 |
37 |
0 |
|
Decreased leukocytes |
71 |
36 |
9 |
0 |
|
Increased glucose |
66 |
57 |
3 |
3 |
|
Decreased neutrophils |
66 |
25 |
23 |
1 |
|
Decreased lymphocytes |
51 |
29 |
7 |
3 |
|
Increased alkaline phosphatase |
46 |
21 |
1 |
0 |
|
Increased creatinine |
40 |
23 |
0 |
0 |
|
Decreased magnesium |
36 |
34 |
1 |
0 |
|
Increased aspartate aminotransferase |
35 |
17 |
1 |
0.4 |
|
Increased alanine aminotransferase |
29 |
17 |
2 |
1 |
Patients Receiving ZEJULA with Dose Based on Baseline Weight or Platelet Count in PRIMA
Among patients who received ZEJULA with the dose based on weight and platelet count, the median duration of treatment was 11.0 months (range: 1 day to 16 months).
Serious adverse reactions occurred in 27% of patients receiving ZEJULA. Serious adverse reactions in > 2% of patients were anemia (8%), and thrombocytopenia (7%). No fatal adverse reactions occurred.
Permanent discontinuation due to adverse reactions occurred in 14% of patients who received ZEJULA. Adverse reactions resulting in permanent discontinuation in >2% of patients who received ZEJULA included thrombocytopenia and anemia (3.0% each), and nausea (2.4%).
Adverse reactions led to dose reduction or interruption in 72% of patients, most frequently from thrombocytopenia (40%), anemia (23%), and neutropenia (15%).
Table 6 and Table 7 summarize adverse reactions and abnormal laboratory findings in the group of patients who received ZEJULA.
| Grades 1-4b | Grades 3-4b | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZEJULA
N=169 % | Placebo
N=86 % | ZEJULA
N=169 % | Placebo
N=86 % |
|||||||||||
| aAll adverse reactions in the table consist of grouped preferred terms except for nausea, vomiting, decreased appetite, headache and insomnia, which are single preferred terms. bCTCAE=Common Terminology Criteria for Adverse Events version 4.02 cincludes neutropenia, neutropenic infection, neutropenic sepsis, febrile neutropenia. dincludes leukopenia, lymphocyte count decreased, lymphopenia, white blood cell count decreased. eincludes blood creatinine increased, blood urea increased, acute kidney injury, renal failure, blood creatine increased. |
||||||||||||||
|
Blood and Lymphatic System Disorders |
||||||||||||||
|
Thrombocytopenia |
54 |
5 |
21 |
1 |
||||||||||
|
Anemia |
50 |
28 |
23 |
1 |
||||||||||
|
Neutropeniac |
36 |
8 |
15 |
1 |
||||||||||
|
Leukopeniad |
28 |
11 |
5 |
0 |
||||||||||
|
Gastrointestinal Disorders |
||||||||||||||
|
Nausea |
53 |
21 |
1 |
0 |
||||||||||
|
Constipation |
31 |
15 |
1 |
1 |
||||||||||
|
Vomiting |
17 |
9 |
0 |
1 |
||||||||||
|
General Disorders and Administration Site Conditions |
||||||||||||||
|
Fatigue |
48 |
36 |
3 |
0 |
||||||||||
|
Metabolism and Nutrition Disorders |
||||||||||||||
|
Decreased appetite |
19 |
5 |
1 |
0 |
||||||||||
|
Nervous System Disorders |
||||||||||||||
|
Headache |
22 |
17 |
1 |
0 |
||||||||||
|
Dizziness |
14 |
13 |
0 |
0 |
||||||||||
|
Psychiatric Disorders |
||||||||||||||
|
Insomnia |
21 |
14 |
0 |
0 |
||||||||||
|
Renal and Urinary Disorders |
||||||||||||||
|
Acute kidney injurye |
12 |
5 |
1 |
0 |
||||||||||
|
Respiratory, Thoracic and Mediastinal Disorders |
||||||||||||||
|
Dyspnea |
18 |
10 |
0 |
1 |
||||||||||
|
Vascular Disorders |
||||||||||||||
|
Hypertension |
17 |
9 |
5 |
2 |
||||||||||
| Grades 1-4 | Grades 3-4 | |||
|---|---|---|---|---|
| ZEJULA
N=169 % | Placebo
N=86 % | ZEJULA
N=169 % | Placebo
N=86 % |
|
|
Decreased hemoglobin |
81 |
70 |
21 |
0 |
|
Decreased leukocytes |
70 |
36 |
6 |
0 |
|
Decreased platelets |
63 |
15 |
18 |
0 |
|
Increased glucose |
63 |
56 |
2 |
1 |
|
Decreased neutrophils |
60 |
27 |
15 |
0 |
|
Decreased lymphocytes |
52 |
30 |
5 |
4 |
|
Increased alkaline phosphatase |
43 |
17 |
1 |
0 |
|
Decreased magnesium |
44 |
30 |
0 |
0 |
|
Increased creatinine |
41 |
22 |
0 |
0 |
|
Increased aspartate aminotransferase |
31 |
19 |
1 |
0 |
|
Increased alanine aminotransferase |
28 |
15 |
2 |
2 |
Maintenance Treatment of Recurrent Ovarian Cancer
The safety of ZEJULA monotherapy 300 mg once daily has been studied in 367 patients with platinum-sensitive recurrent ovarian, fallopian tube, and primary peritoneal cancer in the NOVA trial. Adverse reactions in NOVA led to dose reduction or interruption in 69% of patients, most frequently from thrombocytopenia (41%) and anemia (20%). The permanent discontinuation rate due to adverse reactions in NOVA was 15%. The median exposure to ZEJULA in these patients was 250 days.
Table 8 and Table 9 summarize the common adverse reactions and abnormal laboratory findings, respectively, observed in patients treated with ZEJULA in NOVA.
| Grades 1-4a | Grades 3-4a | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZEJULA
N=367 % | Placebo
N=179 % | ZEJULA
N=367 % | Placebo
N=179 % |
|||||||||||||||||||||
| aCTCAE=Common Terminology Criteria for Adverse Events version 4.02 bincludes preferred terms of neutropenic infection, neutropenic sepsis, and febrile neutropenia. |
||||||||||||||||||||||||
|
Blood and Lymphatic System Disorders |
||||||||||||||||||||||||
|
Thrombocytopenia |
61 |
5 |
29 |
0.6 |
||||||||||||||||||||
|
Anemia |
50 |
7 |
25 |
0 |
||||||||||||||||||||
|
Neutropeniab |
30 |
6 |
20 |
2 |
||||||||||||||||||||
|
Leukopenia |
17 |
8 |
5 |
0 |
||||||||||||||||||||
|
Cardiac Disorders |
||||||||||||||||||||||||
|
Palpitations |
10 |
2 |
0 |
0 |
||||||||||||||||||||
|
Gastrointestinal Disorders |
||||||||||||||||||||||||
|
Nausea |
74 |
35 |
3 |
1 |
||||||||||||||||||||
|
Constipation |
40 |
20 |
0.8 |
2 |
||||||||||||||||||||
|
Vomiting |
34 |
16 |
2 |
0.6 |
||||||||||||||||||||
|
Mucositis/stomatitis |
20 |
6 |
0.5 |
0 |
||||||||||||||||||||
|
Dyspepsia |
18 |
12 |
0 |
0 |
||||||||||||||||||||
|
Dry mouth |
10 |
4 |
0.3 |
0 |
||||||||||||||||||||
|
General Disorders and Administration Site Conditions |
||||||||||||||||||||||||
|
Fatigue/Asthenia |
57 |
41 |
8 |
0.6 |
||||||||||||||||||||
|
Metabolism and Nutrition Disorders |
||||||||||||||||||||||||
|
Decreased appetite |
25 |
15 |
0.3 |
0.6 |
||||||||||||||||||||
|
Infections and Infestations |
||||||||||||||||||||||||
|
Urinary tract infection |
13 |
8 |
0.8 |
1 |
||||||||||||||||||||
|
Investigations |
||||||||||||||||||||||||
|
AST/ ALT elevation |
10 |
5 |
4 |
2 |
||||||||||||||||||||
|
Musculoskeletal and Connective Tissue Disorders |
||||||||||||||||||||||||
|
Back pain |
18 |
12 |
0.8 |
0 |
||||||||||||||||||||
|
Nervous System Disorders |
||||||||||||||||||||||||
|
Headache |
26 |
11 |
0.3 |
0 |
||||||||||||||||||||
|
Dizziness |
18 |
8 |
0 |
0 |
||||||||||||||||||||
|
Dysgeusia |
10 |
4 |
0 |
0 |
||||||||||||||||||||
|
Psychiatric Disorders |
||||||||||||||||||||||||
|
Insomnia |
27 |
8 |
0.3 |
0 |
||||||||||||||||||||
|
Anxiety |
11 |
7 |
0.3 |
0.6 |
||||||||||||||||||||
|
Respiratory, Thoracic, and Mediastinal Disorders |
||||||||||||||||||||||||
|
Nasopharyngitis |
23 |
14 |
0 |
0 |
||||||||||||||||||||
|
Dyspnea |
20 |
8 |
1 |
1 |
||||||||||||||||||||
|
Cough |
16 |
5 |
0 |
0 |
||||||||||||||||||||
|
Skin and Subcutaneous Tissue Disorders |
||||||||||||||||||||||||
|
Rash |
21 |
9 |
0.5 |
0 |
||||||||||||||||||||
|
Vascular Disorders |
||||||||||||||||||||||||
|
Hypertension |
20 |
5 |
9 |
2 |
||||||||||||||||||||
| N=number of patients; WBC=white blood cells; ALT=Alanine aminotransferase; AST=Aspartate aminotransferase | |||||||||||||||||||||||||||||
|
Grades 1-4 |
Grades 3-4 |
||||||||||||||||||||||||||||
|
ZEJULA N=367 (%) |
Placebo N= 179 (%) |
ZEJULA N= 367 (%) |
Placebo N= 179 (%) |
||||||||||||||||||||||||||
|
Decrease in hemoglobin |
85 |
56 |
25 |
0.5 |
|||||||||||||||||||||||||
|
Decrease in platelet count |
72 |
21 |
35 |
0.5 |
|||||||||||||||||||||||||
|
Decrease in WBC count |
66 |
37 |
7 |
0.7 |
|||||||||||||||||||||||||
|
Decrease in absolute neutrophil count |
53 |
25 |
21 |
2 |
|||||||||||||||||||||||||
|
Increase in AST |
36 |
23 |
1 |
0 |
|||||||||||||||||||||||||
|
Increase in ALT |
28 |
15 |
1 |
2 |
|||||||||||||||||||||||||
The following adverse reactions and laboratory abnormalities have been identified in ≥1 to <10% of the 367 patients receiving ZEJULA in the NOVA trial and not included in the table: tachycardia, peripheral edema, hypokalemia, bronchitis, conjunctivitis, gamma-glutamyl transferase increased, blood creatinine increased, blood alkaline phosphatase increased, weight decreased, depression, epistaxis.
Treatment of Advanced Ovarian Cancer after Three or More Chemotherapies
The safety of ZEJULA monotherapy 300 mg once daily has been studied in QUADRA, a single-arm study in 463 patients with recurrent high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who had been treated with 3 or more prior lines of therapy. The median duration of overall study treatment was 3 months (range: 0.03 to 32 months). For the indicated QUADRA population, the median duration was 4 months (range: 0.1 to 30 months).
Fatal adverse reactions occurred in 2% of patients, including cardiac arrest.
Serious adverse reactions occurred in 43% of patients receiving ZEJULA. Serious adverse reactions in >3% of patients were small intestinal obstruction (7%), vomiting (6%), nausea (5%), and abdominal pain (4%).
Permanent discontinuation due to adverse reactions (Grade 1-4) occurred in 21% of patients who received ZEJULA.
Adverse reactions led to dose reduction or interruption in 73% of patients receiving ZEJULA. The most common adverse reactions (≥5%) resulting in dose reduction or interruption of ZEJULA were thrombocytopenia (40%), anemia (21%), neutropenia (11%), nausea (13%), vomiting (11%), fatigue (9%), and abdominal pain (5%).
Table 10 and Table 11 summarize the common adverse reactions and abnormal laboratory findings, respectively, observed in patients treated with ZEJULA in QUADRA.
| Grades 1-4a
N=463 % | Grades 3-4a
N=463 % |
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aCTCAE=Common Terminology Criteria for Adverse Events version 4.02 bAnemia includes events with preferred terms of anemia, hemoglobin decreased, anemia macrocytic, aplastic anemia, and normochromic normocytic anemia. cThrombocytopenia includes events with preferred terms of thrombocytopenia and platelet count decreased. dNeutropenia includes events with preferred terms of neutropenia, neutrophil count decreased, neutropenic infection and neutropenic sepsis. |
||||||||||||||||||||||||||||||||
|
Blood and Lymphatic System Disorders |
||||||||||||||||||||||||||||||||
|
Anemiab |
51 |
27 |
||||||||||||||||||||||||||||||
|
Thrombocytopeniac |
52 |
28 |
||||||||||||||||||||||||||||||
|
Neutropeniad |
20 |
13 |
||||||||||||||||||||||||||||||
|
Gastrointestinal Disorders |
||||||||||||||||||||||||||||||||
|
Nausea |
67 |
10 |
||||||||||||||||||||||||||||||
|
Vomiting |
44 |
8 |
||||||||||||||||||||||||||||||
|
Constipation |
36 |
5 |
||||||||||||||||||||||||||||||
|
Abdominal pain |
34 |
7 |
||||||||||||||||||||||||||||||
|
Diarrhea |
17 |
0.2 |
||||||||||||||||||||||||||||||
|
General Disorders and Administration Site Conditions |
||||||||||||||||||||||||||||||||
|
Fatigue |
56 |
7 |
||||||||||||||||||||||||||||||
|
Infections and Infestations |
||||||||||||||||||||||||||||||||
|
Urinary tract infection |
15 |
2 |
||||||||||||||||||||||||||||||
|
Investigations |
||||||||||||||||||||||||||||||||
|
Blood alkaline phosphatase increased |
11 |
2 |
||||||||||||||||||||||||||||||
|
AST/ALT elevation |
11 |
1 |
||||||||||||||||||||||||||||||
|
Metabolism and Nutrition Disorders |
||||||||||||||||||||||||||||||||
|
Decreased appetite |
27 |
2 |
||||||||||||||||||||||||||||||
|
Musculoskeletal and Connective Tissue Disorders |
||||||||||||||||||||||||||||||||
|
Musculoskeletal pain |
29 |
3 |
||||||||||||||||||||||||||||||
|
Nervous System Disorders |
||||||||||||||||||||||||||||||||
|
Headache |
19 |
0.4 |
||||||||||||||||||||||||||||||
|
Dizziness |
11 |
0 |
||||||||||||||||||||||||||||||
|
Psychiatric Disorders |
||||||||||||||||||||||||||||||||
|
Insomnia |
21 |
1 |
||||||||||||||||||||||||||||||
|
Renal and Urinary Disorders |
||||||||||||||||||||||||||||||||
|
Acute kidney injury |
17 |
1 |
||||||||||||||||||||||||||||||
|
Respiratory, Thoracic and Mediastinal Disorders |
||||||||||||||||||||||||||||||||
|
Dyspnea |
22 |
3 |
||||||||||||||||||||||||||||||
|
Cough |
13 |
0 |
||||||||||||||||||||||||||||||
|
Vascular Disorders |
||||||||||||||||||||||||||||||||
|
Hypertension |
14 |
5 |
||||||||||||||||||||||||||||||
| Grades 1-4
N=463 % | Grades 3-4
N=463 % |
|
|---|---|---|
|
Decreased hemoglobin |
83 |
26 |
|
Increased glucose |
66 |
5 |
|
Decreased platelets |
60 |
28 |
|
Decreased lymphocytes |
57 |
18 |
|
Decreased leukocytes |
53 |
9 |
|
Decreased magnesium |
46 |
1 |
|
Increased alkaline phosphatase |
40 |
4 |
|
Increased gamma glutamyl transferase |
40 |
8 |
|
Increased creatinine |
36 |
0.4 |
|
Decreased sodium |
34 |
6 |
|
Decreased neutrophils |
34 |
15 |
|
Increased aspartate aminotransferase |
29 |
2 |
|
Decreased albumin |
27 |
2 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ZEJULA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: hypersensitivity (including anaphylaxis)
Nervous System Disorders: posterior reversible encephalopathy syndrome (PRES)
Psychiatric Disorders: confusional state/disorientation, hallucination, cognitive impairment
Respiratory, Thoracic, and Mediastinal Disorders: non-infectious pneumonitis
Skin and Subcutaneous Tissue Disorders: photosensitivity
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, ZEJULA can cause fetal harm when administered to pregnant women [see Clinical Pharmacology (12.1)]. There are no data regarding the use of ZEJULA in pregnant women to inform the drug-associated risk. ZEJULA has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow) [see Warnings and Precautions (5.2) and Nonclinical Toxicology (13.1)]. Due to the potential risk to a fetus based on its mechanism of action, animal developmental and reproductive toxicology studies were not conducted with niraparib. Apprise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
No data are available regarding the presence of niraparib or its metabolites in human milk, or on its effects on the breastfed infant or milk production. Because of the potential for serious adverse reactions in breastfed infants from ZEJULA, advise a lactating woman not to breastfeed during treatment with ZEJULA and for 1 month after receiving the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
ZEJULA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
A pregnancy test is recommended for females of reproductive potential prior to initiating ZEJULA treatment.
Contraception
Females
ZEJULA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with ZEJULA and for at least for 6 months following the last dose.
Infertility
Males
Based on animal studies, ZEJULA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of ZEJULA have not been established in pediatric patients.
8.5 Geriatric Use
In PRIMA, 39% of patients were aged ≥65 years and 10% were aged ≥75 years. In NOVA, 35% of patients were aged ≥65 years and 8% were aged ≥75 years. No overall differences in safety and effectiveness of ZEJULA were observed between these patients and younger patients but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dose adjustment is necessary for patients with mild (CLcr:60 to 89 mL/min) to moderate (CLcr:30 to 59 mL/min) renal impairment. The degree of renal impairment was determined by creatinine clearance as estimated by the Cockcroft-Gault equation. The safety of ZEJULA in patients with severe renal impairment or end stage renal disease undergoing hemodialysis is unknown.
10 OVERDOSAGE
There is no specific treatment in the event of ZEJULA overdose, and symptoms of overdose are not established. In the event of an overdose, healthcare practitioners should follow general supportive measures and should treat symptomatically.
11 DESCRIPTION
Niraparib is an orally available poly(ADP-ribose) polymerase (PARP) inhibitor.
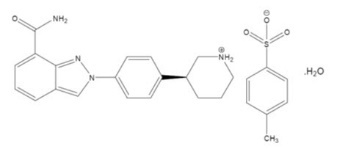
The chemical name for niraparib tosylate monohydrate is 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole 7-carboxamide 4-methylbenzenesulfonate hydrate (1:1:1). The molecular formula is C26H30N4O5S and it has a molecular weight of 510.61 amu. The molecular structure is shown below:
Niraparib tosylate monohydrate is a white to off-white, non-hygroscopic crystalline solid. Niraparib solubility is pH independent below the pKa of 9.95, with an aqueous free base solubility of 0.7 mg/mL to 1.1 mg/mL across the physiological pH range.
Each ZEJULA capsule contains 159.4 mg niraparib tosylate monohydrate equivalent to 100 mg niraparib free base as the active ingredient. The inactive ingredients in the capsule fill are magnesium stearate and lactose monohydrate. The capsule shell consists of titanium dioxide, gelatin in the white capsule body; and FD&C Blue #1, FD&C Red #3, FD&C Yellow #5 and gelatin in the purple capsule cap. The black printing ink consists of shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide and black iron oxide. The white printing ink consists of shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, sodium hydroxide, povidone and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Niraparib is an inhibitor of poly(ADP-ribose) polymerase (PARP) enzymes, PARP-1 and PARP-2, which play a role in DNA repair. In vitro studies have shown that niraparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis and cell death. Increased niraparib-induced cytotoxicity was observed in tumor cell lines with or without deficiencies in BRCA1/2. Niraparib decreased tumor growth in mouse xenograft models of human cancer cell lines with deficiencies in BRCA1/2 and in human patient-derived xenograft tumor models with homologous recombination deficiency that had either mutated or wild type BRCA1/2.
12.2 Pharmacodynamics
The pharmacodynamic response of niraparib has not been characterized.
Cardiovascular Effects
Niraparib has the potential to cause effects on pulse rate and blood pressure in patients receiving the recommended dose, which may be related to pharmacological inhibition of the dopamine transporter (DAT), norepinephrine transporter (NET) and serotonin transporter (SERT) [see Nonclinical Toxicology (13.2)].
In the PRIMA study, mean pulse rate and blood pressure increased over baseline in the niraparib arm relative to the placebo arm at most on-study assessments. Mean greatest increases from baseline in pulse rate on treatment were 22.4 and 14.0 beats/min in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in systolic blood pressure on treatment were 24.4 and 19.6 mmHg in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in diastolic blood pressure on treatment were 15.9 and 13.9 mmHg in the niraparib and placebo arms, respectively.
In the NOVA study, mean pulse rate and blood pressure increased over baseline in the niraparib arm relative to the placebo arm at all on-study assessments. Mean greatest increases from baseline in pulse rate on treatment were 24.1 and 15.8 beats/min in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in systolic blood pressure on treatment were 24.5 and 18.3 mmHg in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in diastolic blood pressure on treatment were 16.5 and 11.6 mmHg in the niraparib and placebo arms, respectively.
Cardiac Electrophysiology
The potential for QTc prolongation with niraparib was evaluated in a randomized, placebo-controlled trial in cancer patients (367 patients on niraparib and 179 patients on placebo). No large changes in the mean QTc interval (>20 ms) were detected in the trial following the treatment of niraparib 300 mg once daily.
12.3 Pharmacokinetics
Following a single-dose administration of 300 mg niraparib, the mean (±SD) peak plasma concentration (Cmax) was 804 (± 403) ng/mL. The exposure (Cmax and AUC) of niraparib increased in a dose proportional manner with daily doses ranging from 30 mg (0.1 times the approved recommended dosage) to 400 mg (1.3 times the approved recommended dosage). The accumulation ratio of niraparib exposure following 21 days of repeated daily doses was approximately 2 fold for doses ranging from 30 mg to 400 mg.
Absorption
The absolute bioavailability of niraparib is approximately 73%. Following oral administration of niraparib, peak plasma concentration, Cmax, is reached within 3 hours.
Concomitant administration of a high fat meal (800-1,000 calories with approximately 50% of total caloric content of the meal from fat) did not significantly affect the pharmacokinetics of niraparib.
Distribution
Niraparib is 83.0% bound to human plasma proteins. The average (±SD) apparent volume of distribution (Vd/F) was 1220 (±1114) L. In a population pharmacokinetic analysis, the Vd/F of niraparib was 1074 L in cancer patients.
Elimination
Following multiple daily doses of 300 mg niraparib, the mean half-life (t1/2) is 36 hours. In a population pharmacokinetic analysis, the apparent total clearance (CL/F) of niraparib was 16.2 L/h in cancer patients.
Metabolism
Niraparib is metabolized by carboxylesterases (CEs) to form a major inactive metabolite, which subsequently undergoes glucuronidation.
Excretion
Following administration of a single oral 300 mg dose of radio-labeled niraparib, the average percent recovery of the administered dose over 21 days was 47.5% (range 33.4% to 60.2%) in urine, and 38.8% (range 28.3% to 47.0%) in feces. In pooled samples collected over 6 days, unchanged niraparib accounted for 11% and 19% of the administered dose recovered in urine and feces, respectively.
Specific Populations
Age (18 to 65 years old), race/ethnicity, mild to moderate renal impairment, and mild hepatic impairment had no clinically significant effect on the pharmacokinetics of niraparib.
The effect of severe renal impairment or end-stage renal disease undergoing hemodialysis on the pharmacokinetics of niraparib is unknown.
The effect of moderate or severe hepatic impairment on the pharmacokinetics of niraparib is unknown.
Drug Interaction Studies
No clinical drug interaction studies have been performed with ZEJULA.
In Vitro Studies
- Inhibition of CYPs: Neither niraparib nor the major primary metabolite M1 is an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4.
- Induction of CYPs: Neither niraparib nor M1 is a CYP3A4 inducer. Niraparib weakly induces CYP1A2 in vitro.
- Substrate of CYPs: Niraparib is a substrate of carboxylesterases (CEs) and the resulting M1 is further metabolized through the formation of glucuronides in vivo.
- Inhibition of UGTs: Niraparib did not exhibit inhibitory effect against the UGT isoforms (UGT1A1, UGT1A4, UGT1A9, and UGT2B7) up to 200 µM in vitro. Therefore, the potential for a clinically relevant inhibition of UGTs by niraparib is minimal.
- Inhibition of transporter systems: Niraparib is a weak inhibitor of breast cancer resistance protein (BCRP), but does not inhibit P-glycoprotein (P-gp), bile salt export pump (BSEP), or multidrug resistance-associated protein 2 (MRP2).
- Niraparib is an inhibitor of multidrug and toxin extrusion (MATE)1 and 2 with IC50 of 0.18 µM and ≤ 0.14 µM, respectively. Increased plasma concentrations of co-administered drugs that are substrates of these transporters (e.g., metformin) cannot be excluded.
- The M1 metabolite is not an inhibitor of P-gp, BCRP, BSEP, MRP2, or MATE1 or 2. Neither niraparib nor M1 is an inhibitor of organic anion transport polypeptide 1B1 (OATP1B1), 1B3 (OATP1B3), or organic cation transporter 1 (OCT1), organic anion transporter 1 (OAT1), organic anion transporter 3 (OAT3), or organic cation transporter 2 (OCT2).
- Substrate of transporter systems: Niraparib is a substrate of P-gp and BCRP. Niraparib is not a substrate of BSEP, MRP2, or MATE1 or 2. The M1 metabolite is not a substrate of P-gp, BCRP, BSEP, or MRP2, but MATE1 and 2. Neither niraparib nor M1 is a substrate of OATP1B1, OATP1B3, OCT1, OAT1, OAT3, or OCT2.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with niraparib.
Niraparib was clastogenic in an in vitro mammalian chromosomal aberration assay and in an in vivo rat bone marrow micronucleus assay. This clastogenicity is consistent with genomic instability resulting from the primary pharmacology of niraparib and indicates potential for genotoxicity in humans. Niraparib was not mutagenic in a bacterial reverse mutation assay (Ames) test.
Fertility studies in animals have not been conducted with niraparib. In repeat-dose oral toxicity studies, niraparib was administered daily for up to 3 months duration in rats and dogs. Reduced sperm, spermatids and germ cells in epididymides and testes were observed at doses ≥10 mg/kg and ≥1.5 mg/kg in rats and dogs, respectively. These dose levels resulted in systemic exposures approximately 0.3 and 0.012 times, respectively, the human exposure (AUC0-24hr) at the recommended dose of 300 mg daily. There was a trend toward reversibility of these findings 4 weeks after dosing was stopped.
13.2 Animal Toxicology and/or Pharmacology
In vitro, niraparib bound to the dopamine transporter (DAT), norepinephrine transporter (NET) and serotonin transporter (SERT) and inhibited uptake of norepinephrine and dopamine in cells with IC50 values that were lower than the Cmin at steady-state in patients receiving the recommended dose. Niraparib has the potential to cause effects in patients related to inhibition of these transporters (e.g., cardiovascular or CNS).
Intravenous administration of niraparib to vagotomized dogs over 30 minutes at 1, 3 and 10 mg/kg resulted in an increased range of arterial pressures of 13-20, 18-27 and 19-25% and increased range of heart rates of 2-11, 4-17 and 12-21% above pre-dose levels, respectively. The unbound plasma concentrations of niraparib in dogs at these dose levels were approximately 0.5, 1.5 and 5.8 times the unbound Cmax at steady-state in patients receiving the recommended dose.
In addition, niraparib crossed the blood-brain barrier in rats and monkeys following oral administration. The cerebrospinal fluid (CSF):plasma Cmax ratios of niraparib administered at 10 mg/kg orally to two Rhesus monkeys were 0.10 and 0.52.
14 CLINICAL STUDIES
14.1 First-Line Maintenance Treatment of Advanced Ovarian Cancer
PRIMA (NCT02655016) was a double-blind, placebo-controlled trial in which patients (n=733) in complete or partial response to first-line platinum-based chemotherapy were randomized 2:1 to ZEJULA or matched placebo. Initially, the patients received a starting dose of 300 mg once daily, regardless of body weight or platelet count. The study was amended to include a starting dose of 200 mg for patients weighing less than 77 kg OR with a platelet count of less than 150,000/μL or 300 mg for patients weighing greater than or equal to 77 kg (170 lbs) AND who have a platelet count greater than or equal to 150,000/μL.
Patients were randomized post completion of first-line platinum-based chemotherapy plus surgery. Randomization was stratified by best response during the front-line platinum regimen (complete response vs partial response), neoadjuvant chemotherapy (NACT) (yes vs no), and HRD status (positive vs negative or not determined). HRD status was determined using the FDA-approved Myriad myChoice CDx assay. HRD positive status included either tumor BRCA mutant (tBRCAm) or a genomic instability score (GIS) ≥ 42.
The major efficacy outcome measure, progression-free survival (PFS), was determined by blinded independent central review (BICR) per RECIST, version 1.1. In some cases, criteria other than RECIST, such as clinical signs and symptoms and increasing CA-125, were also applied. Overall survival (OS) was an additional efficacy outcome measure. PFS testing was performed hierarchically: first in the HR deficient (HRD positive) population, then in the overall population. The median age of 62 ranged from 32 to 85 years among patients randomized with ZEJULA and 33 to 88 years among patients randomized with placebo. Eighty-nine percent of all patients were white. Sixty-nine percent of patients randomized with ZEJULA and 71% of patients randomized with placebo had an ECOG of 0 at study baseline. Approximately 45% of patients were enrolled in the U.S. or Canada. In the overall population, 65% of patients had stage III disease and 35% had stage IV disease. Sixty-seven percent of the patients received NACT. Sixty-nine percent of the patients had a complete response to the first-line platinum-based chemotherapy. Approximately 35% (n=258) of patients received a starting dose of 200 mg or 300 mg depending on baseline body weight and platelet count. Among those patients, 186 patients received a starting dose of 200 mg.
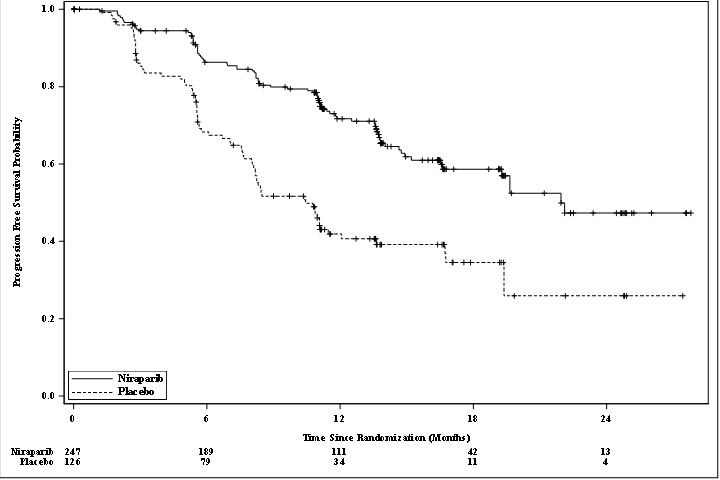
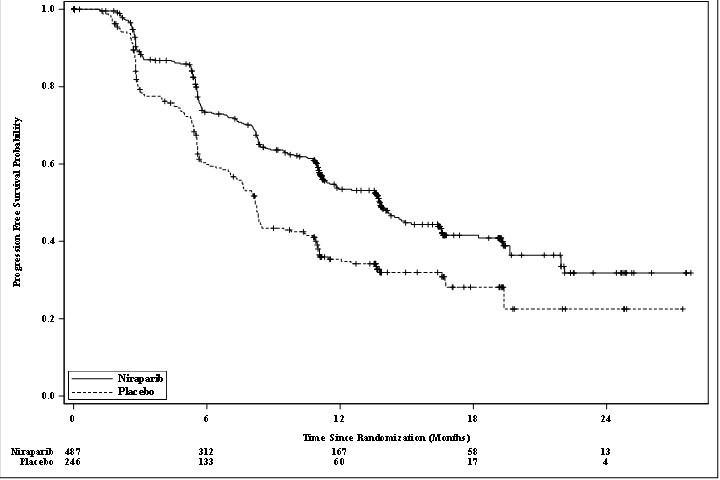
PRIMA demonstrated a statistically significant improvement in PFS for patients randomized to ZEJULA as compared with placebo in the HR deficient and overall population (Table 12, and Figures 1 and 2).
| aefficacy analysis was based on blinded independent central review (BICR). bbased on a stratified Cox proportional hazards model cbased on a stratified log-rank test NE=Not Estimable |
||||
|
HR deficient Population |
Overall Population |
|||
|
ZEJULA (N=247) |
Placebo (N=126) |
ZEJULA (N=487) |
Placebo (N=246) |
|
|
PFS events, n (%) |
81 (33) |
73 (58) |
232 (48) |
155 (63) |
|
PFS Median (95% CI), in months |
21.9 (19.3, NE) |
10.4 (8.1, 12.1) |
13.8 (11.5, 14.9) |
8.2 (7.3, 8.5) |
|
Hazard Ratio (HR)b (95% CI) |
0.43 (0.31, 0.59) |
0.62 (0.50, 0.76) |
||
|
p-valuec |
<0.0001 |
<0.0001 |
||
In exploratory subgroup analyses of patients who were administered a starting dose of ZEJULA or matched placebo based on baseline weight or platelet count, the hazard ratio for PFS was 0.39 (95% CI [0.22, 0.72]) in the HR deficient subgroup (n=130), and 0.68 (95% CI [0.48, 0.97]) in the overall population (n=258).
Figure 1: Progression-Free Survival in Patients with HR deficient Tumors (ITT Population, N=373)
Figure 2: Progression-Free Survival in the Overall Population (ITT Population, N=733)
At the time of the PFS analysis, overall survival data were immature with 11% deaths in the overall population.
14.2 Maintenance Treatment of Recurrent Ovarian Cancer
NOVA (NCT01847274) was a double-blind, placebo-controlled trial in which patients (n=553) with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer were randomized 2:1 to ZEJULA 300 mg orally daily or matched placebo within 8 weeks of the last therapy. Treatment was continued until disease progression or unacceptable toxicity. All patients had received at least two prior platinum-containing regimens and were in response (complete or partial) to their most recent platinum-based regimen.
Randomization was stratified by time to progression after the penultimate platinum therapy (6 to <12 months and ≥12 months); use of bevacizumab in conjunction with the penultimate or last platinum regimen (yes/no); and best response during the most recent platinum regimen (complete response and partial response). Eligible patients were assigned to one of two cohorts based on the results of the BRACAnalysis CDx. Patients with deleterious or suspected deleterious germline BRCA mutations (gBRCAm) were assigned to the germline BRCA mutated (gBRCAmut) cohort (n=203), and those without germline BRCA mutations were assigned to the non-gBRCAmut cohort (n=350).
The major efficacy outcome measure, PFS (progression-free survival), was determined primarily by central independent assessment per RECIST (Response Evaluation Criteria in Solid Tumors, version 1.1). In some cases, criteria other than RECIST, such as clinical signs and symptoms and increasing CA-125, were also applied.
The median age of patients ranged from 57-64 years among patients treated with ZEJULA and 58-67 years among patients treated with placebo. Eighty-six percent of all patients were white. Sixty-seven percent of patients receiving ZEJULA and 69% of patients receiving placebo had an ECOG of 0 at study baseline. Approximately 40% of patients were enrolled in the U.S. or Canada and 51% of all patients were in complete response to most recent platinum-based regimen, with 39% on both arms with an interval of 6-12 months since the penultimate platinum regimen. Twenty-six percent of those treated with ZEJULA and 31% treated with placebo had received prior bevacizumab therapy. Approximately 40% of patients had 3 or more lines of treatment.
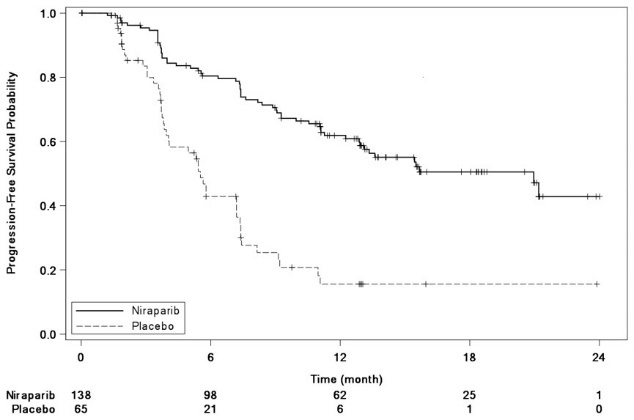
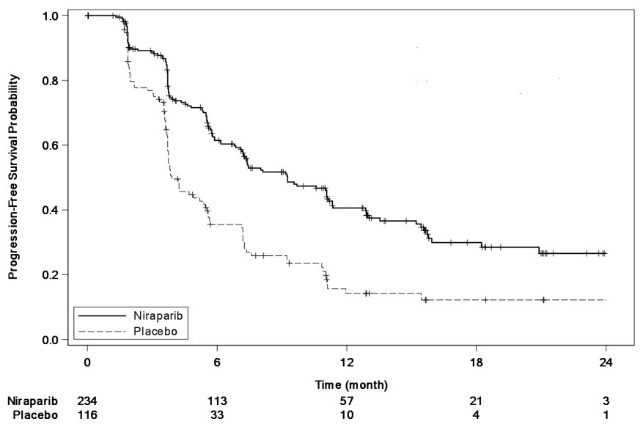
The trial demonstrated a statistically significant improvement in PFS for patients randomized to ZEJULA as compared with placebo in the gBRCAmut cohort and the non-gBRCAmut cohort (Table 13, and Figures 3 and 4).
| gBRCAmut Cohort | non-gBRCAmut Cohort | |||
|---|---|---|---|---|
| ZEJULA
(N=138) | Placebo (N=65) | ZEJULA
(N=234) | Placebo (N=116) | |
| aefficacy analysis was based on blinded central independent radiologic and clinical oncology review committee (IRC). bbased on a stratified Cox proportional hazards model cbased on a stratified log-rank test NR=Not Reached |
||||
|
PFS Median in months (95% CI) |
21.0
|
5.5
|
9.3
|
3.9
|
|
Hazard Ratio (HR)b
|
0.26
|
0.45
|
||
|
p-valuec |
<0.0001 |
<0.0001 |
||
Figure 3: Progression-Free Survival in the gBRCAmut Cohort Based on IRC Assessment (ITT Population, N=203)
Figure 4: Progression-Free Survival in the Non-gBRCAmut Cohort Overall Based on IRC Assessment (ITT Population, N=350)
At the time of the PFS analysis, limited overall survival data were available with 17% deaths across the two cohorts.
14.3 Treatment of Advanced Ovarian Cancer after Three or More Chemotherapies
The efficacy of ZEJULA was studied in 98 patients with advanced ovarian cancer with HRD positive tumors in the single-arm QUADRA (NCT02354586) trial. Patients were required to have been treated with three or more prior lines of chemotherapy and those with prior exposure to PARP inhibitors were excluded. Patients were selected using a clinical trial assay. Those without BRCA mutations must have progressed at least six months after their last dose of platinum therapy. All patients received ZEJULA capsules at a starting dose of 300 mg once daily as monotherapy until disease progression or unacceptable toxicity.
HRD positive status was determined using the Myriad myChoice CDx as either tBRCAm (n=63) and/or a genomic instability score (GIS) ≥ 42 (n=35). GIS is an algorithmic measurement of Loss of Heterozygosity (LOH), Telomeric Allelic Imbalance (TAI), and Large-scale State Transitions (LST).
The major efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) as assessed by the investigator according to RECIST v. 1.1.
The median age of the patients was 63 years (range 39-91); the majority were white (82%) and all had an ECOG PS of 0 (59%) or 1 (41%).
The efficacy results for QUADRA are summarized in Table 14.
| HRD Positive Cohorta | |
|---|---|
| N=98 | |
| aHRD positive status is defined as tBRCA-mutated and/or GIS > 42. bConfirmed response rate. The ORR as assessed by blinded independent central review was consistent. cNE=not estimable. |
|
|
Objective Response Rate (95% CI)b |
24% (16, 34) |
|
Complete Responses |
0% |
|
Partial Responses |
24% |
|
Median DOR in months (95% CI) |
8.3 (6.5, NEc) |
For patients with tBRCAm ovarian cancer, investigator-assessed ORR was 39% (7/18; 95% CI: [17, 64]) in patients with platinum-sensitive disease, 29% (6/21; 95% CI: [11, 52]) in patients with platinum-resistant disease, and 19% (3/16; 95% CI: [4, 46]) in patients with platinum-refractory disease.
For patients with platinum-sensitive GIS-positive disease (without BRCAmut) (n=35), investigator-assessed ORR was 20% (95% CI [8, 37]).
16 HOW SUPPLIED/STORAGE AND HANDLING
ZEJULA is available as capsules having a white body printed with “100 mg” in black ink, and a purple cap printed with “Niraparib” in white ink.
Each capsule contains 100 mg of niraparib free base.
ZEJULA capsules are packaged as
|
90-count bottles |
NDC 69656-103-90 |
|
30-count bottles |
NDC 69656-103-30 |
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
MDS/AML
Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts, or a need for blood transfusions. This may be a sign of hematological toxicity or myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) which has been reported in patients treated with ZEJULA [see Warnings and Precautions (5.1)].
Bone Marrow Suppression
Advise patients that periodic monitoring of their blood counts is required. Advise patients to contact their healthcare provider for new onset of bleeding, fever, or symptoms of infection [see Warnings and Precautions (5.2)].
Cardiovascular Effects
Advise patients to undergo blood pressure and heart rate monitoring at least weekly for the first two months, then monthly for the first year of treatment, and then periodically thereafter. Advise patients to contact their healthcare provider if blood pressure is elevated [see Warnings and Precautions (5.3)].
Dosing Instructions
Inform patients on how to take ZEJULA [see Dosage and Administration (2.2)]. ZEJULA should be taken once daily. Instruct patients that if they miss a dose of ZEJULA, not to take an extra dose to make up for the one that they missed. They should take their next dose at the regularly scheduled time. Each capsule should be swallowed whole. ZEJULA may be taken with or without food. Bedtime administration may be a potential method for managing nausea.
Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1)].
Contraception
Advise females of reproductive potential to use effective contraception during treatment with ZEJULA and for at least 6 months after receiving the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise patients not to breastfeed while taking ZEJULA and for 1 month after the last dose [see Use in Special Populations (8.2)].
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured for GlaxoSmithKline
Research Triangle Park, NC 27709
©2020 GSK group of companies. 124212
|
PATIENT INFORMATION
|
|||
|
What is the most important information I should know about ZEJULA? |
|||
|
ZEJULA may cause serious side effects including: |
|||
|
|||
|
|
||
|
Your healthcare provider will do blood tests to check your blood cell counts: |
|||
|
|||
|
|||
|
See “What are the possible side effects of ZEJULA?” for more information about side effects. |
|||
|
What is ZEJULA? |
|||
|
ZEJULA is a prescription medicine used for the: |
|||
|
|||
It is not known if ZEJULA is safe and effective in children. |
|||
|
Before taking ZEJULA, tell your healthcare provider about all of your medical conditions, including if you: |
|||
|
|||
|
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|||
|
How should I take ZEJULA? |
|||
|
|||
|
What are the possible side effects of ZEJULA? |
|||
|
ZEJULA can cause serious side effects, including: |
|||
|
|||
|
The most common side effects of ZEJULA include: |
|||
|
|
||
|
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with ZEJULA, if you have certain side effects. |
|||
|
How should I store ZEJULA?
|
|||
|
General information about the safe and effective use of ZEJULA. |
|||
|
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZEJULA for a condition for which it was not prescribed. Do not give ZEJULA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ZEJULA that is written for health professionals. |
|||
|
What are the ingredients in ZEJULA? |
|||
|
Active ingredient: niraparib |
|||
|
Inactive ingredients: |
|||
|
Capsule fill: magnesium stearate and lactose monohydrate The black printing ink: shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide and black iron oxide. Trademarks are owned by or licensed to the GSK group of companies. |
|||
|
Manufactured for GlaxoSmithKline Research Triangle Park, NC 27709 ©2020 GSK group of companies. 124212 For more information, call 1-888-825-5249 or go to www.gsk.com |
|||
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: April 2020
PRINCIPAL DISPLAY PANEL
NDC 69656-103-30
Zejula
(niraparib)
capsules
100 mg
Rx only
30 capsules
Each 100-mg capsule is equivalent to 159.4 mg of niraparib tosylate monohydrate.
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F), [See USP Controlled Room Temperature]
Do not accept if membrane seal under cap is missing or broken.
See prescribing information for dosage information.
Keep out of reach of children.
Trademarks are owned by or licensed to the GSK group of companies.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2020 GSK group of companies or its licensor.
Rev.2/20
123648
| ZEJULA
niraparib capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - GlaxoSmithKline LLC (167380711) |