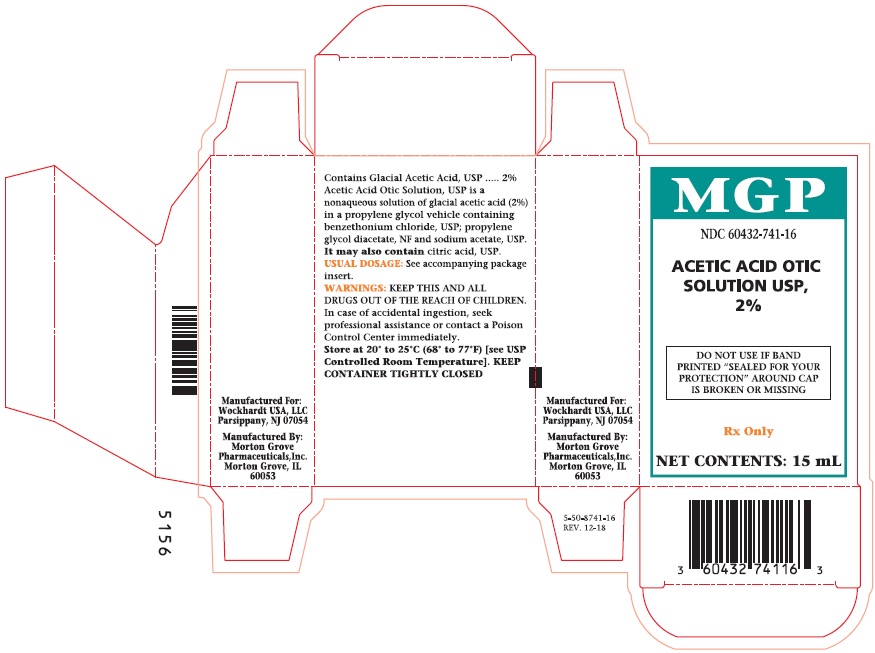
Label: ACETIC ACID solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 60432-741-15, 60432-741-16 - Packager: Morton Grove Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 4, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Acetic Acid Otic Solution, USP is a nonaqueous solution of glacial acetic acid, USP (2%), in a propylene glycol vehicle containing benzethonium chloride, USP (0.02%); propylene glycol diacetate, NF (3%) and sodium acetate, USP (0.015%). It may also contain citric acid, USP.
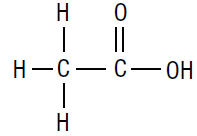
The molecular formula for acetic acid is CH3COOH, with a molecular weight of 60.05. The structural formula is:
Acetic Acid Otic Solution is available as a nonaqueous otic solution buffered at pH 3 for use in the external ear canal.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Carefully remove all cerumen and debris to allow Acetic Acid Otic Solution to contact infected surfaces directly. To promote continuous contact, insert a wick of cotton saturated with the solution into the ear canal; the wick may also be saturated after insertion. Instruct the patient to keep the wick in for at least 24 hours and to keep it moist by adding 3 to 5 drops of the solution every 4 to 6 hours. The wick may be removed after 24 hours but the patient should continue to instill 5 drops of Acetic Acid Otic Solution 3 or 4 times daily thereafter, for as long as indicated. In pediatric patients, 3 to 4 drops may be sufficient due to the smaller capacity of the ear canal.
-
HOW SUPPLIED
Acetic Acid Otic Solution USP, 2% is supplied in 15 mL measured drop, safety-tip plastic bottles.
RECOMMENDED STORAGE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
KEEP CONTAINER TIGHTLY CLOSED
Rx Only
Product No.: 8741
Manufactured For:
Wockhardt USA, LLC
Parsippany, NJ 07054
Manufactured By:
Morton Grove Pharmaceuticals, Inc
Morton Grove, IL 60053
A50-8741-15
REV. 09-12
-
PRINCIPAL DISPLAY PANEL
NDC 60432-741-15
ACETIC ACID
OTIC
SOLUTION
USP, 2%
DO NOT USE IF BAND
PRINTED "SEALED FOR YOUR
PROTECTION" AROUND CAP
IS BROKEN OR MISSING.
Rx Only
NET CONTENTS:
15 mL

NDC 60432-741-16
ACETIC ACID
OTIC
SOLUTION
USP, 2%
DO NOT USE IF BAND
PRINTED "SEALED FOR YOUR
PROTECTION" AROUND CAP
IS BROKEN OR MISSING.
Rx Only
NET CONTENTS:
15 mL
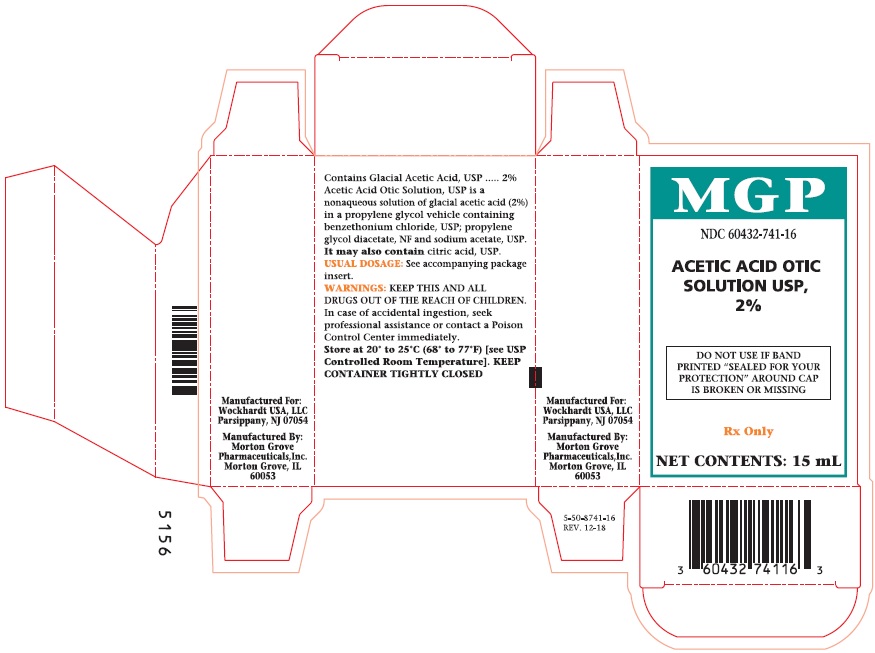
Acetic Acid Carton
-
INGREDIENTS AND APPEARANCE
ACETIC ACID
acetic acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60432-741 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLENE GLYCOL DIACETATE (UNII: 5Z492UNF9O) BENZETHONIUM CHLORIDE (UNII: PH41D05744) SODIUM ACETATE (UNII: 4550K0SC9B) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60432-741-15 15 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/26/1996 2 NDC:60432-741-16 1 in 1 CARTON 01/04/2019 2 NDC:60432-741-15 15 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040166 07/26/1996 Labeler - Morton Grove Pharmaceuticals, Inc. (801897505) Registrant - Morton Grove Pharmaceuticals, Inc. (801897505) Establishment Name Address ID/FEI Business Operations Morton Grove Pharmaceuticals, Inc. 801897505 ANALYSIS(60432-741) , LABEL(60432-741) , MANUFACTURE(60432-741) , PACK(60432-741)