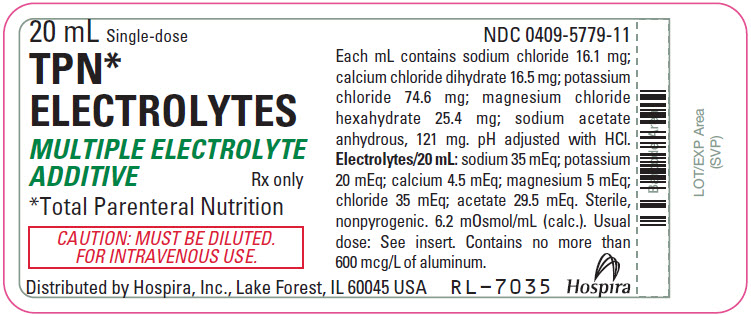
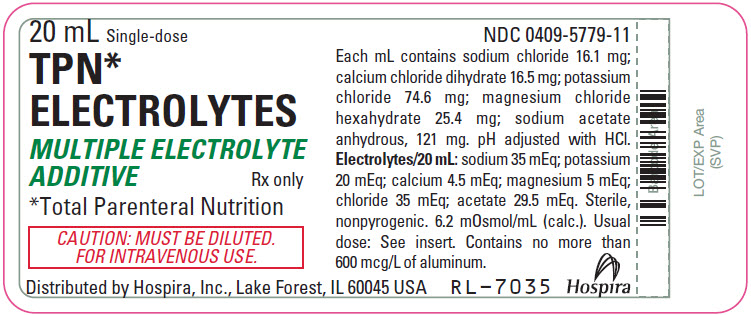
Label: TPN ELECTROLYTES- sodium chloride, calcium chloride, potassium chloride, magnesium chloride, and sodium acetate anhydrous injection, solution, concentrate
- NDC Code(s): 0409-5779-01, 0409-5779-11
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
TPN Electrolytes (multiple electrolyte additive) is a sterile, nonpyrogenic, concentrated solution of intra- and extracellular ions for intravenous infusion after dilution as a maintenance electrolyte replenisher only. It contains no phosphate and no bacteriostat, antimicrobial agent or added buffer. The pH is 6.6 (6.0 to 7.5). May contain hydrochloric acid for pH adjustment. The osmolar concentration is 6.2 mOsmol/mL (calc.).
__________________________
*Total Parenteral Nutrition
Ingredients and ion constituents of the solution are as follows:
Ingredient or Ion
mg/20 mL
mEq/20 mL
Sodium Chloride
321
Calcium Chloride (dihydrate)
331
Potassium Chloride
1491
Magnesium Chloride (hexahydrate)
508
Sodium Acetate (anhydrous)
2420
Sodium (Na+)
35
Potassium (K+)
20
Calcium (Ca++)
4.5
Magnesium (Mg++)
5
Chloride (Cl−)
35
Acetate (CH3COO−)
29.5
Sodium Chloride, USP is chemically designated NaCl, a white crystalline compound freely soluble in water.
Calcium Chloride, USP dihydrate is chemically designated CaCl2 • 2H2O, white, odorless fragments or granules, freely soluble in water.
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Magnesium Chloride, USP hexahydrate is chemically designated MgCl2 • 6H2O, deliquescent crystals very soluble in water.
Sodium Acetate, USP anhydrous is chemically designated C2H3NaO2, a hygroscopic powder very soluble in water.
Water for Injection, USP is chemically designated H2O.
The semi-rigid container is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
-
CLINICAL PHARMACOLOGY
TPN Electrolytes (multiple electrolyte additive) helps to maintain normal cellular metabolism during TPN (total parenteral nutrition). Providing electrolytes in appropriate amounts prevents deficiency symptoms which otherwise would occur in their absence.
Cations: Sodium is the principal extracellular cation; it helps maintain motor nerve depolarization, proper fluid balance and normal renal metabolism. Potassium is the principal intracellular cation; it helps transport dextrose across the cell membrane and contributes to normal renal function. Magnesium is an important cofactor for enzymatic reactions and helps to maintain normal CNS (central nervous system) activity and amino acid utilization. Calcium participates in muscle contraction, blood coagulation and helps maintain normal neuromuscular function.
Anions: Chloride is the principal extracellular anion which, along with bicarbonate, is involved in maintaining proper anion balance. Acetate is an important metabolic intermediate in the tricarboxylic acid cycle and is a bicarbonate alternate.
The distribution and excretion of sodium (Na+) and chloride (Cl−) are largely under the control of the kidney which maintains a balance between intake and output.
Approximately 80% of body calcium (Ca++) is excreted in the feces as insoluble salts; urinary excretion accounts for the remaining 20%.
Potassium (K+) is found in low concentration in the plasma and extracellular fluids (3.5 to 5.0 mEq/liter in a healthy adult). Normally about 80% to 90% of the potassium intake is excreted in the urine, the remainder in the stools and to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting or in patients on a potassium-free diet, potassium loss from the body continues resulting in potassium depletion.
Magnesium (Mg++) is the second most plentiful intracellular cation. Normal plasma concentration ranges from 1.5 to 2.5 or 3.0 mEq per liter. Magnesium is excreted solely by the kidney at a rate proportional to the plasma concentration and glomerular filtration.
Acetate (CH3COO−) provides bicarbonate (HCO3−) by metabolic conversion in the liver. This has been shown to proceed readily even in the presence of severe liver disease.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
TPN Electrolytes (multiple electrolyte additive) is contraindicated in pathological conditions where additives of potassium, sodium, calcium, magnesium or chloride could be clinically deleterious, e.g., anuria, hyperkalemia, heart block or myocardial damage and severe edema due to cardiovascular, renal or hepatic failure.
-
WARNINGS
CONCENTRATED, HYPERTONIC, ADDITIVE SOLUTION. Must be diluted in TPN solution prior to administration.
CONTAINS NO PHOSPHATE. Patients receiving TPN solutions containing concentrated dextrose require additive phosphate, in addition to TPN Electrolytes. Between 10 and 15 mM (310 to 465 mg) phosphorus are physically compatible with as much as 10 to 12 mEq calcium in the same admixture. The phosphate supplement should first be added to the amino acid or dextrose bottle and diluted well to avoid precipitation with calcium.
CONTAINS 20 mEq of POTASSIUM. The potassium content of other additives, such as potassium phosphate or potassium-containing antibiotics, must be considered in the context of total potassium delivered. TPN patients usually require 30 to 50 mEq of potassium per liter of TPN solution containing concentrated (20—25%) dextrose.
NOT INTENDED FOR PEDIATRIC USE.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
Solutions which contain potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is present.
In patients with diminished renal function, administration of solutions containing sodium or potassium ions may result in sodium or potassium retention.
Solutions containing acetate ions should be used with great care in patients with metabolic or respiratory alkalosis. Acetate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
Warning: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Blood levels of sodium, potassium, calcium, magnesium, phosphorus and chloride should be monitored frequently during TPN (total parenteral nutrition). Significant deviations from normal may justify further supplementation or substitution of individual electrolyte additives (in place of TPN Electrolytes) to tailor the electrolyte supplement to meet individual patient requirements.
In patients with renal dysfunction or cardiovascular insufficiency, especially in elderly or postsurgical patients, consider the potential effects of sodium (35 mEq) and potassium (20 mEq) present in TPN Electrolytes.
Extraordinary electrolyte losses are not necessarily corrected by TPN Electrolytes. In protracted vomiting or diarrhea or in patients with fistula drainage or nasogastric suction, separate replacement therapy may be necessary, based upon analysis of losses sustained.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Solutions containing acetate ions should be used with caution as excess administration may result in metabolic alkalosis.
Pregnancy
Animal reproduction studies have not been conducted with TPN Electrolytes. It is also not known whether TPN Electrolytes can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. TPN Electrolytes should be given to a pregnant woman only if clearly needed.
GERIATRIC USE
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Sodium ions and phosphorus are known to be substantially secreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Symptoms may result from an excess or deficit of one or more of the ions present in TPN Electrolytes. Therefore, frequent monitoring of electrolyte blood levels is recommended. Sodium excess can cause edema and exacerbation of congestive heart failure. Excess potassium can cause deviations from the normal ECG (electrocardiogram). Potassium deficits can impair neuromuscular function, causing muscle weakness or frank paralysis, intestinal dilatation and ileus. Calcium deficits can produce neuromuscular hyperexcitability, ranging from paresthesias, cramps and laryngospasm to tetany and grand mal seizures. Depressed calcium levels can accompany administration of parenteral phosphorous or large amounts of albumin. Magnesium deficiency can precipitate neuromuscular dysfunction, hyperirritability, psychotic behavior, tachycardia and hypertension. Magnesium excess can cause muscle weakness, ECG changes, sedation and mental confusion.
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
One 20 mL volume of TPN Electrolytes (multiple electrolyte additive) is added to each liter of amino acid/dextrose solution. Alternatively, the TPN Electrolytes can be added to the bottle of amino acids or concentrated dextrose, to permit addition of the necessary phosphate additive to the remaining bottle. This latter technique helps avoid physical incompatibilities between calcium and phosphorus. A potassium phosphate additive is recommended for addition to nutritional solutions containing TPN Electrolytes. Between 10 and 30 mEq of potassium (as phosphate) should be added per liter of TPN solution, to augment the 20 mEq of potassium provided by TPN Electrolytes.
Between two and three liters of TPN solution with added TPN Electrolytes are usually administered daily to adults. Solutions are given continuously over the entire 24-hour period at a constant rate, ranging from 83 to 125 mL/hour. TPN solutions containing TPN Electrolytes and concentrated dextrose are administered intravenously, through a central venous catheter.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
-
HOW SUPPLIED
TPN Electrolytes (multiple electrolyte additive) is supplied in the following single-dose delivery system:
Unit of Sale Concentration Each NDC 0409-5779-01
25 in a carton20 mL
NDC 0409-5779-11
20 mL VialExposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1091-1.0
Revised: 9/2017
- PRINCIPAL DISPLAY PANEL - 20 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 20 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
TPN ELECTROLYTES
sodium chloride, calcium chloride, potassium chloride, magnesium chloride, and sodium acetate anhydrous injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-5779 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 321 mg in 20 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 331 mg in 20 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 1491 mg in 20 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 508 mg in 20 mL SODIUM ACETATE ANHYDROUS (UNII: NVG71ZZ7P0) (SODIUM CATION - UNII:LYR4M0NH37, ACETATE ION - UNII:569DQM74SC) SODIUM ACETATE ANHYDROUS 2420 mg in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-5779-01 25 in 1 CARTON 02/28/2005 1 NDC:0409-5779-11 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018895 02/28/2005 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-5779) , LABEL(0409-5779) , MANUFACTURE(0409-5779) , PACK(0409-5779) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-5779) Establishment Name Address ID/FEI Business Operations Macco Organiques, S.r.o. 521632476 API MANUFACTURE(0409-5779) Establishment Name Address ID/FEI Business Operations Hospira Worldwide, LLC 963711309 ANALYSIS(0409-5779)