CEFOTETAN AND DEXTROSE- cefotetan and dextrose injection, solution
B. Braun Medical Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CEFOTETAN for injection AND DEXTROSE injection safely and effectively. See full prescribing information for CEFOTETAN for injection AND DEXTROSE injection.
CEFOTETAN for injection AND DEXTROSE injection, for intravenous use Initial U.S. Approval: 1985 INDICATIONS AND USAGECefotetan for Injection and Dextrose Injection is a cephalosporin antibacterial indicated for the treatment of the following infections caused by susceptible isolates of the designated bacteria (1):
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefotetan for Injection and Dextrose Injection and other antibacterial drugs, Cefotetan for Injection and Dextrose Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.8) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence > 1%): Gastrointestinal, including diarrhea. Hematologic abnormalities. Hepatic enzyme elevations. Hypersensitivity reactions. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact B. Braun Medical Inc. at 1-800-854-6851 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 1/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Urinary Tract Infections
Cefotetan for Injection and Dextrose Injection is indicated for the treatment of urinary tract infections caused by E. coli, Klebsiella spp (including K. pneumoniae), Proteus mirabilis, Proteus vulgaris, Providencia rettgeri, and Morganella morganii.
1.2 Lower Respiratory Tract Infections
Cefotetan for Injection and Dextrose Injection is indicated for the treatment of lower respiratory tract Infections caused by Streptococcus pneumoniae, Staphylococcus aureus (methicillin susceptible), Haemophilus influenzae, Klebsiella species (including K. pneumoniae), E. coli, Proteus mirabilis, and Serratia marcescens.
1.3 Skin and Skin Structure Infections
Cefotetan for Injection and Dextrose Injection is indicated for the treatment of skin and skin structure infections due to Staphylococcus aureus (methicillin susceptible), Staphylococcus epidermidis (methicillin susceptible), Streptococcus pyogenes, Streptococcus species, Escherichia coli, Klebsiella pneumoniae, Peptococcus niger, Peptostreptococcus species.
1.4 Gynecologic Infections
Cefotetan for Injection and Dextrose Injection is indicated for the treatment of gynecologic Infections caused by Staphylococcus aureus (methicillin susceptible), Staphylococcus epidermidis (methicillin susceptible), Streptococcus species, Streptococcus agalactiae, E. coli, Proteus mirabilis, Neisseria gonorrhoeae, Bacteroides fragilis, Prevotella melaninogenica, Bacteroides vulgatus, Fusobacterium species, and gram-positive anaerobic cocci (including Peptococcus niger and Peptostreptococcus species).
Cefotetan has no activity against Chlamydia trachomatis. Therefore, when cefotetan is used in the treatment of pelvic inflammatory disease, and C. trachomatis is one of the suspected pathogens, appropriate antichlamydial coverage should be added.
1.5 Intra-abdominal Infections
Cefotetan for Injection and Dextrose Injection is indicated for the treatment of intra-abdominal Infections caused by E. coli, Klebsiella species (including K. pneumoniae), Streptococcus species, Bacteroides fragilis, Prevotella melaninogenica, Bacteroides vulgatus, and Clostridium species (other than Clostridium difficile [see Warnings and Precautions (5.4)].
1.6 Bone and Joint Infections
Cefotetan for Injection and Dextrose Injection is indicated for the treatment of bone and joint infections caused by Staphylococcus aureus (methicillin susceptible).
1.7 Prophylaxis
The preoperative administration of Cefotetan for Injection and Dextrose Injection may reduce the incidence of certain postoperative infections in patients undergoing surgical procedures that are classified as clean, contaminated or potentially contaminated (e.g., cesarean section, abdominal or vaginal hysterectomy, transurethral surgery, biliary tract surgery, and gastrointestinal surgery).
If there are signs and symptoms of infection, specimens for culture should be obtained for identification of the causative organism so that appropriate therapeutic measures may be initiated.
1.8 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefotetan for Injection and Dextrose Injection and other antibacterial drugs, Cefotetan for Injection and Dextrose Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antimicrobial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Adult Treatment
The usual adult dosage is 1 gram (g) or 2 grams of Cefotetan for Injection and Dextrose Injection administered intravenously (IV) every 12 hours for 5 to 10 days. Proper dosage should be determined by the condition of the patient, severity of the infection, and susceptibility of the causative organism.
See Table 1. Use this formulation of cefotetan only in patients who require the entire 1 or 2 gram dose and not any fraction thereof.
| Table 1: Recommended Dosing Schedule of Cefotetan for Injection and Dextrose Injection in Patients with Normal Renal Function | ||
| Type of Infection | Daily Dose | Frequency and Route |
| Urinary Tract | 1-4 grams |
1 or 2 g every 24 hours IV 1 or 2 g every 12 hours IV |
| Skin & Skin Structure | ||
| Mild – Moderate* | 2 grams | 2 g every 24 hours IV |
| 1 g every 12 hours IV | ||
| Severe | 4 grams | 2 g every 12 hours IV |
| Other Sites | 2-4 grams | 1 or 2 g every 12 hours IV |
| Severe | 4 grams | 2 g every 12 hours IV |
| Life-Threatening | 6 grams† | 3 g every 12 hours IV |
2.2 Adult Prophylaxis
To prevent postoperative infection in clean, contaminated, or potentially contaminated surgery in adults, the recommended dosage is 1 or 2 g of Cefotetan for Injection and Dextrose Injection administered once, intravenously, 30 to 60 minutes prior to surgery. In patients undergoing cesarean section, the dose should be administered as soon as the umbilical cord is clamped.
2.3 Patients with Impaired Renal Function
When renal function is impaired, a reduced dosage schedule must be employed. The dosage guidelines in Table 2 may be used.
|
||
| Table 2: Recommended Dosing Schedule for Cefotetan for Injection and Dextrose Injection in Adult Patients with Impaired Renal Function | ||
| Creatinine Clearance | ||
| mL/min | Dose | Frequency |
| Greater than 30 | Usual Recommended Dosage* | Every 12 hours |
| 10 to 30 | Usual Recommended Dosage* | Every 24 hours |
| Less than 10 | Usual Recommended Dosage* | Every 48 hours |
Alternatively, the dosing interval may remain constant at 12 hour intervals, but the dose reduced to one-half the usual recommended dose for patients with a creatinine clearance of 10 to 30 mL/min, and one-quarter the usual recommended dose for patients with a creatinine clearance of less than 10 mL/min.
When only serum creatinine levels are available, creatinine clearance may be calculated from the following formula. The serum creatinine level should represent a steady state of renal function.
Males: Weight (kg) x (140 - age)
72 x serum creatinine (mg/100 mL)
Females: 0.85 x value for males
Cefotetan is dialyzable and it is recommended that for patients undergoing intermittent hemodialysis, one-quarter of the usual recommended dose be given every 24 hours on days between dialysis and one-half the usual recommended dose on the day of dialysis.
2.4 Preparation and Administration of Cefotetan for Injection and Dextrose Injection in DUPLEX® Container
Important Administration Instructions
• Do not use in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete. If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result.
• Do not introduce additives into the DUPLEX® Container.
• Administer Cefotetan for Injection and Dextrose Injection intravenously over approximately 30 minutes.
• Using an infusion system, Cefotetan for Injection and Dextrose Injection may be administered over a longer period of time through the tubing system by which the patient may be receiving other intravenous solutions. Butterfly or scalp vein-type needles are preferred for this type of infusion. However, during infusion of the solution containing Cefotetan for Injection and Dextrose Injection, it is advisable to discontinue temporarily the administration of other solutions at the same site.
• Cefotetan for Injection and Dextrose Injection must not be admixed with solutions containing aminoglycosides. If Cefotetan for Injection and Dextrose Injection and aminoglycosides are to be administered to the same patient, they must be administered separately and not as a mixed injection.
• This reconstituted solution is for intravenous use only.
• Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
• Use only if solution is clear and container and seals are intact.
DUPLEX® CONTAINER Storage
• To avoid inadvertent activation, DUPLEX® Container should remain in the folded position until activation is intended.
Patient Labeling and Drug Powder/Diluent Inspection
• Apply patient-specific label on foil side of container. Use care to avoid activation. Do not cover any portion of foil strip with patient label.
• Unlatch side tab and unfold DUPLEX® Container (see Diagram 1).
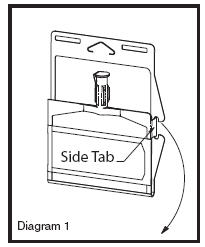
• Visually inspect diluent chamber for particulate matter.
• Use only if container and seals are intact.
• To inspect the drug powder for foreign matter or discoloration, peel foil strip from drug chamber (see Diagram 2).
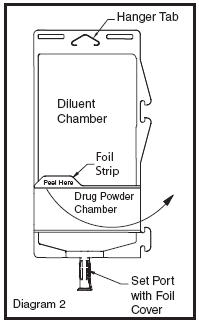
• Protect from light after removal of foil strip.
• Note: If foil strip is removed, the container should be re-folded and the side tab latched until ready to activate. The product must then be used within 7 days, but not beyond the labeled expiration date.
Reconstitution (Activation)
• Do not use directly after storage by refrigeration, allow the product to equilibrate to room temperature before patient use.
• Unfold the DUPLEX® Container and point the set port in a downward direction. Starting at the hanger tab end, fold the DUPLEX® Container just below the diluent meniscus trapping all air above the fold. To activate, squeeze the folded diluent chamber until the seal between the diluent and powder opens, releasing diluent into the drug powder chamber (see Diagram 3).
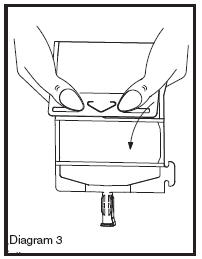
• Agitate the liquid-powder mixture until the drug powder is completely dissolved.
• Following reconstitution (activation), product must be used within 12 hours if stored at room temperature or within 5 days if stored under refrigeration.
• Reconstituted Cefotetan for Injection and Dextrose Injection tends to darken depending on storage conditions, within the stated recommendations. However, product potency is not adversely affected.
• Use only if prepared solution is clear and free from particulate matter.
Administration
• Visually inspect the reconstituted solution for particulate matter.
• Point the set port in a downwards direction. Starting at the hanger tab end, fold the DUPLEX® Container just below the solution meniscus trapping all air above the fold. Squeeze the folded DUPLEX® Container until the seal between reconstituted drug solution and set port opens, releasing liquid to set port (see Diagram 4).
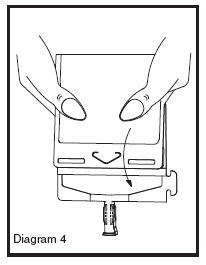
• Prior to attaching the IV set, check for minute leaks by squeezing container firmly. If leaks are found, discard container and solution as sterility may be compromised.
• Using aseptic technique, peel foil cover from the set port and attach sterile administration set (see Diagram 5).
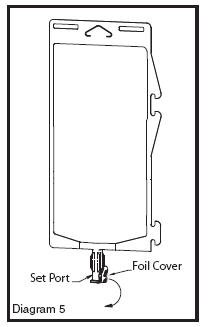
• Refer to Directions for Use accompanying the administration set.
3 DOSAGE FORMS AND STRENGTHS
Single-dose DUPLEX® Container consisting of:
• 1 g cefotetan for injection USP, as a dry, white to pale yellow powder, and 50 mL of 5% dextrose injection USP, as a clear solution.
• 2 g cefotetan for injection USP, as a dry, white to pale yellow powder, and 50 mL of 5% dextrose injection USP, as a clear solution.
4 CONTRAINDICATIONS
4.1 Hypersensitivity to Cefotetan, or Other Beta-lactam Antibacterial Drugs
Cefotetan for Injection and Dextrose Injection is contraindicated in patients who have shown hypersensitivity to cefotetan or to other beta-lactam antibacterial drugs (e.g., penicillins and cephalosporins) [see Warnings and Precautions (5.1)].
4.2 Cephalosporin-associated hemolytic anemia
Cefotetan for Injection and Dextrose Injection is contraindicated in patients who have experienced a cephalosporin associated hemolytic anemia [see Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions to Cefotetan, or Other Beta-lactam Antibacterial Drugs
Serious and occasionally fatal hypersensitivity reactions (e.g., anaphylaxis) have been reported in patients on beta-lactam antibacterials, including cefotetan [see Adverse Reactions (6.1)]. These reactions are more likely to occur in individuals with a history of beta-lactam hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Cefotetan for Injection and Dextrose Injection is contraindicated in patients with a known hypersensitivity to cefotetan or other beta-lactam antibacterial drugs [see Contraindications (4)]. Before initiating therapy with Cefotetan for Injection and Dextrose Injection, inquire about previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. Cross-hypersensitivity among beta-lactam antibacterial drugs has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue Cefotetan for Injection and Dextrose Injection and institute appropriate therapy.
5.2 Hemolytic Anemia
An immune mediated hemolytic anemia has been observed in patients receiving cephalosporin class antibacterials. Severe cases of hemolytic anemia, including fatalities, have been reported in association with the administration of Cefotetan for Injection and Dextrose Injection. There appears to be an increased risk of developing hemolytic anemia on Cefotetan for Injection and Dextrose Injection relative to other cephalosporins of at least 3 fold. If a patient develops anemia anytime within 2–3 weeks subsequent to the administration of Cefotetan for Injection and Dextrose Injection, the diagnosis of a cephalosporin associated anemia should be considered and the drug stopped until the etiology is determined with certainty. Blood transfusions may be considered as needed [see Contraindications (4.2)].
Patients who receive courses of Cefotetan for Injection and Dextrose Injection for the treatment or prophylaxis of infections should have periodic monitoring for signs and symptoms of hemolytic anemia including a measurement of hematological parameters where appropriate.
5.3 Prothrombin Activity and Risk of Bleeding
Cefotetan for Injection and Dextrose Injection may be associated with a fall in prothrombin activity and, possibly, subsequent bleeding. Those at increased risk include patients with renal or hepatobiliary impairment or poor nutritional state, the elderly, and patients with cancer. Prothrombin time should be monitored and exogenous vitamin K administered as indicated.
5.4 Clostridium difficile-associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Cefotetan for Injection and Dextrose Injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.5 Risk of Development of Drug-resistant Bacteria
Prescribing Cefotetan for Injection and Dextrose Injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Prolonged use of Cefotetan for Injection and Dextrose Injection may result in overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection does occur during therapy, appropriate measures should be taken.
5.6 Drug/Laboratory Test Interactions
Urinary Glucose
The administration of Cefotetan for Injection and Dextrose Injection may result in a false positive reaction for glucose in the urine when using glucose tests based on Benedict’s copper reduction reaction that determine the amount of reducing substances like glucose in the urine. It is recommended that glucose tests based on enzymatic glucose oxidase be used.
5.7 Patients with a History of Gastrointestinal Disease
Cefotetan for Injection and Dextrose Injection is not recommended in individuals with a history of gastrointestinal disease, particularly colitis.
5.8 Patients with Overt or Known Subclinical Diabetes Mellitus or Carbohydrate Intolerance
Cefotetan for Injection and Dextrose Injection should be monitored in patients if Cefotetan for Injection and Dextrose Injection is prescribed in patients with overt or known subclinical diabetes mellitus or carbohydrate intolerance for any reason.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
• Hypersensitivity reactions to Cefotetan, Cephalosporins, Penicillins, or Other Beta-lactam Antibacterial Drugs [see Warnings and Precautions (5.1)]
• Hemolytic Anemia [see Warnings and Precautions (5.2)]
• Prothrombin Activity and Risk of Bleeding [see Warnings and Precautions (5.3)]
• Clostridium difficile-associated diarrhea [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In clinical studies, the following adverse reactions were considered related to Cefotetan for Injection and Dextrose Injection therapy.
| Table 3: Adverse Reactions (≥1%) in Clinical Studies of Cefotetan | |
| Adverse Reaction |
Cefotetan (%) |
| Gastrointestinal disorders | |
| Diarrhea | 1.25% |
| Hematologic disorders | |
| Laboratory abnormalities | 1.4% |
| Hepatic enzyme elevations | 1.2% |
| Immune System disorders | |
| Hypersensitivity reactions | 1.2% |
| Table 4: Adverse Reactions (<1%) in Clinical Studies of Cefotetan | |
| Adverse Reaction |
Cefotetan (%) |
| Gastrointestinal disorders | |
| Nausea | 0.14% |
| Hematologic disorders | |
| Eosinophilia | 0.5% |
| Positive direct Coombs’ test | 0.4% |
| Thrombocytosis | 0.3% |
| Hepatic enzyme elevations | |
| Elevation in ALT (SGPT) | 0.7% |
| Elevation in AST (SGOT) | 0.3% |
| Elevation in alkaline phosphatase | 0.14% |
| Elevation in LDH | 0.14 % |
| Immune System disorders | |
| Rash | 0.7% |
| Itching | 0.14% |
| Local Reactions | |
| Phlebitis at site of injection | 0.3% |
| Discomfort | 0.2% |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Cefotetan for Injection and Dextrose Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to readily estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal: pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment or surgical prophylaxis. [see Warnings and Precautions (5.4)].
Hematologic: agranulocytosis, hemolytic anemia, leukopenia, thrombocytopenia, and prolonged prothrombin time with or without bleeding.
Hypersensitivity: anaphylactic reactions and urticaria.
Renal: Elevations in BUN and serum creatinine have been reported.
Urogenital: Nephrotoxicity has been reported.
Miscellaneous: Fever
6.3 Cephalosporin-class Adverse Reactions
In addition to the adverse reactions listed above which have been observed in patients treated with cefotetan, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibacterials: pruritus, Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, vomiting, abdominal pain, colitis, superinfection, vaginitis including vaginal candidiasis, renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemorrhage, elevated bilirubin, pancytopenia, and neutropenia.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment, when the dosage was not reduced. [see Dosage and Administration (2) and Overdosage (10)]. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
7 DRUG INTERACTIONS
7.1 Aminoglycosides
Increases in serum creatinine have occurred when Cefotetan for Injection and Dextrose Injection was given alone. If Cefotetan for Injection and Dextrose Injection and an aminoglycoside are used concomitantly, renal function should be carefully monitored, because nephrotoxicity may be potentiated.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published observational studies and case reports over several decades with cephalosporin use, including cefotetan, in pregnant women have not established a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes (see Data). In animal reproduction studies, no adverse developmental effects were observed when pregnant rats and monkeys were administered cefotetan, during the period of organogenesis, at doses up to approximately 3 and 2 times the maximum recommended human dose (MRHD), respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Maternal gonorrhea may be associated with preterm birth, low neonatal birth weight, chorioamnionitis, intrauterine growth restriction, small for gestational age and premature rupture of membranes. Perinatal transmission of gonorrhea to the offspring can result in infant blindness, joint infections and bloodstream infections.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from case-control studies and case reports over several decades have not identified an association with cephalosporin use, including cefotetan, during pregnancy, and major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Cefotetan administered for prophylaxis at the time of delivery has not been associated with any adverse reactions in the infant. Cefotetan crosses the placenta and is present in the amniotic fluid. Available studies have methodologic limitations, including small sample size, retrospective data collection, and inconsistent comparator groups.
Animal Data
Reproduction studies have been performed with cefotetan administered during the period of organogenesis in rats (gestational days 7-17) and monkeys (gestational days 20-45) at doses up to 2000 and 600 mg/kg/day (3 and 2 times the MRHD on a body surface area basis, respectively), and have revealed no evidence of harm to the fetus due to cefotetan. In monkeys, treatment with 600 mg/kg/day was associated with maternal toxicity, including reduced body weight gain, reduced food intake, and increased water consumption. The maternal toxicity in the monkeys appeared to be associated with an increase in spontaneous abortions.
8.2 Lactation
Risk Summary
Published literature reports that cefotetan is present in human milk at low levels following intravenous administration. There is no information regarding effects of cefotetan on milk production or the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for cefotetan and any potential adverse effects on the breastfed child from cefotetan or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of this formulation in pediatric patients have not been established.
8.5 Geriatric Use
Of the 925 subjects who received cefotetan in clinical studies, 492 (53%) were 60 years and older, while 76 (8%) were 80 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and the other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.3), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Information on overdosage with Cefotetan for Injection and Dextrose Injection in humans is not available. If overdosage should occur, it should be treated symptomatically and hemodialysis considered, particularly if renal function is compromised.
11 DESCRIPTION
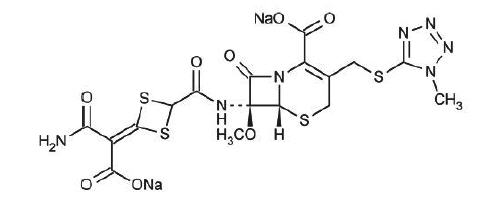
The drug chamber is filled with cefotetan disodium, a sterile, semisynthetic, cephalosporin (cephamycin) antibacterial drug for intravenous administration. Cefotetan disodium is the disodium salt of [6R-(6α,7α)]-7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. The molecular formula of cefotetan disodium is C17H15N7Na2O8S4 with a molecular weight of 619.59. Cefotetan disodium has the following structural formula:
Cefotetan for Injection USP and Dextrose Injection USP in the DUPLEX® container is supplied as a sterile, nonpyrogenic, single use packaged combination of cefotetan disodium and dextrose injection (diluent) in the DUPLEX® sterile container. The DUPLEX® container is a flexible dual chamber container.
Cefotetan disodium is supplied as a dry powder form equivalent to either 1 g or 2 g of cefotetan. Cefotetan disodium is a white to pale yellow powder which is readily soluble in dextrose injection diluent provided in the DUPLEX® container. The pH of freshly reconstituted solution is between 4 to 6.5. The color of reconstituted solutions ranges from colorless to yellow depending on the length of storage and concentration. Cefotetan for Injection USP and Dextrose Injection USP contains approximately 80 mg (3.5 mEq) of sodium per gram of cefotetan activity.
The diluent chamber contains dextrose injection. The concentration of Hydrous Dextrose in Water for Injection USP has been adjusted to render the reconstituted drug product iso-osmotic. Dextrose USP has been added to adjust the osmolality to approximately 290 mOsmol/kg (approximately
1.79 g [3.58% w/v] and 1.04 g [2.08% w/v] to the 1 g and 2 g dosages, respectively). Dextrose injection USP is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents.
Hydrous Dextrose USP has the following structural (molecular) formula:
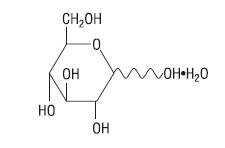
The molecular weight of Hydrous Dextrose USP is 198.17.
After removing the peelable foil strip, activating the seals, and thoroughly mixing, the reconstituted drug product is intended for single intravenous use. When reconstituted according to instructions in the product labeling, the approximate osmolality of the reconstituted solution of Cefotetan for Injection USP and Dextrose Injection USP is about 290 mOsmol/kg.
The DUPLEX® dual chamber container is made from a specially formulated material. The product (diluent and drug) contact layer is a mixture of thermoplastic rubber and a polypropylene ethylene copolymer that contains no plasticizers. The safety of the container system is supported by USP biological evaluation procedures.
Not made with natural rubber latex, PVC or Di(2-ethylhexyl)phthalate (DEHP).
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
High plasma levels of cefotetan are attained after intravenous administration of single doses to normal volunteers.
|
|||||||
|
Table 5: PLASMA CONCENTRATIONS AFTER |
|||||||
| Time After Injection | |||||||
| Route | 15 min | 30 min | 1h | 2h | 4h | 8h | 12h |
| IV | 92 | 158 | 103 | 72 | 42 | 18 | 9 |
|
Table 6: PLASMA CONCENTRATIONS AFTER 2 GRAM IV* DOSE |
|||||||
| Time After Injection | |||||||
| Route | 5 min | 10 min | 1h | 3h | 5h | 9h | 12h |
| IV | 237 | 223 | 135 | 74 | 48 | 22 | 12† |
12.3 Pharmacokinetics
Absorption
Repeated administration of Cefotetan for Injection and Dextrose Injection does not result in accumulation of the drug in normal subjects.
Distribution
Cefotetan is 88% plasma protein bound.
Therapeutic concentrations of cefotetan are achieved in many body tissues and fluids including:
| skin | ureter |
| muscle | bladder |
| fat | maxillary sinus mucosa |
| myometrium | tonsil |
| endometrium | bile |
| cervix | peritoneal fluid |
| ovary | umbilical cord serum |
| kidney | amniotic fluid |
Elimination
Metabolism
The plasma elimination half-life of cefotetan is 3 to 4.6 hours after intravenous administration.
No active metabolites of cefotetan have been detected; however, small amounts (less than 7%) of cefotetan in plasma and urine may be converted to its tautomer, which has antimicrobial activity similar to the parent drug.
Excretion
In normal patients, from 51% to 81% of an administered dose of cefotetan is excreted unchanged by the kidneys over a 24 hour period, which results in high and prolonged urinary concentrations. Following intravenous doses of 1 gram and 2 grams, urinary concentrations are highest during the first hour and reach concentrations of approximately 1700 and 3500 mcg/mL, respectively.
Specific Populations
Geriatric Patients
In pharmacokinetics studies of eight elderly patients (greater than 65 years) with normal renal function and six healthy volunteers (aged 25 to 28 years), mean (±1 SD) Total Body Clearance (1.8 (0.1) L/h vs. 1.8 (0.3) L/h) and mean Volume of Distribution (10.4 (1.2) L vs. 10.3 (1.6) L) were similar following administration of a one gram intravenous bolus dose.
Patients with Renal Impairment
In volunteers with reduced renal function, the plasma half-life of cefotetan is prolonged. The mean terminal half-life increases with declining renal function, from approximately 4 hours in volunteers with normal renal function to about 10 hours in those with moderate renal impairment. There is a linear correlation between the systemic clearance of cefotetan and creatinine clearance. When renal function is impaired, a reduced dosing schedule based on creatinine clearance must be used [see Dosage and Administration (2.3)].
12.4 Microbiology
The bactericidal action of cefotetan results from inhibition of cell wall synthesis. Cefotetan has in vitro activity against a wide range of aerobic and anaerobic gram-positive and gram-negative organisms. The methoxy group in the 7-alpha position provides cefotetan with a high degree of stability in the presence of beta-lactamases including both penicillinases and cephalosporinases of gram-negative bacteria.
Cefotetan has been shown to be active against most isolates of the following organisms both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-Negative Bacteria
Escherichia coli
Haemophilus influenzae (including ampicillin-resistant isolates)
Klebsiella species (including K. pneumoniae)
Morganella morganii
Neisseria gonorrhoeae (nonpenicillinase-producing isolates)
Proteus mirabilis
Proteus vulgaris
Providencia rettgeri
Serratia marcescens
Gram-Positive Bacteria
Staphylococcus aureus (methicillin-susceptible isolates only)
Staphylococcus epidermidis (methicillin-susceptible isolates only)
Streptococcus agalactiae (group B beta-hemolytic streptococcus)
Streptococcus pneumoniae
Streptococcus pyogenes
Streptococcus species
Anaerobes
Prevotella bivia
Prevotella disiens
Bacteroides fragilis
Prevotella melaninogenica
Bacteroides vulgatus
Fusobacterium species
Clostridium species
Peptococcus niger
Peptostreptococcus species
The following in vitro data are available but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for cefotetan against isolates of similar genus or organism group. However, the efficacy of cefotetan in treating clinical infections caused by these bacteria has not been established in clinical trials.
Gram-Negative Bacteria
Citrobacter species (including C. koseri and C. freundii)
Moraxella catarrhalis
Salmonella species
Serratia species
Shigella species
Yersinia enterocolitica
Anaerobes
Porphyromonas asaccharolytica
Prevotella oralis
Bacteroides splanchnicus
Propionibacterium species
Veillonella species
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria, and associated test methods and quality control standards recognized by FDA for this drug, please see: http://www.fda.gov/STIC.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Impairment of Fertility
Cefotetan has adverse effects on the testes of prepubertal rats. Subcutaneous administration of 500 mg/kg/day (approximately 0.8 times the maximum adult human dose on a body surface area basis) on days 6 to 35 of life (thought to be developmentally analogous to late childhood and prepuberty in humans) resulted in reduced testicular weight and seminiferous tubule degeneration in 10 of 10 animals. Affected cells included spermatogonia and spermatocytes; Sertoli and Leydig cells were unaffected. Incidence and severity of lesions were dose-dependent; at 120 mg/kg/day (0.2 times the maximum human dose on a body surface area basis) 1 of 10 treated animals was affected.
Similar lesions have been observed in experiments of comparable design with other methylthiotetrazole-containing antibiotics and impaired fertility has been reported, particularly at high dose levels. No testicular effects were observed in 7-week-old rats treated with up to 1000 mg/kg/day subcutaneous for 5 weeks, or in infant dogs (3 weeks old) that received up to 300 mg/kg/day IV for 5 weeks (both 1.6 times the maximum recommended human dose on a body surface area basis). The relevance of these findings in humans is unknown.
14 CLINICAL STUDIES
Safety and efficacy data for Cefotetan for Injection and Dextrose Injection in the DUPLEX® Container is not available.
16 HOW SUPPLIED/STORAGE AND HANDLING
Cefotetan for Injection USP and Dextrose Injection USP in the DUPLEX® Container is a flexible dual chamber container supplied in two concentrations. After reconstitution, the delivered doses are equivalent to 1 g and 2 g cefotetan. The diluent chamber contains approximately 50 mL of Dextrose Injection. Dextrose Injection has been adjusted to 3.58% w/v and 2.08% w/v for the 1 g and 2 g doses, respectively, such that the reconstituted solution is iso-osmotic.
Cefotetan for Injection USP and Dextrose Injection USP in the DUPLEX® Container is supplied sterile and nonpyrogenic in the DUPLEX® Container packaged 24 units per case.
| NDC | REF | Dose | Volume |
| 0264-3173-11 | 3173-11 | 1 g | 50 mL |
| 0264-3175-11 | 3175-11 | 2 g | 50 mL |
Not made with natural rubber latex.
Store the unactivated unit at 20-25°C (68-77°F). Excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature] Do not freeze.
17 PATIENT COUNSELING INFORMATION
Allergic Reactions
Inform patients that Cefotetan for Injection and Dextrose Injection is a cephalosporin that can cause allergic reactions in some individuals [see Warnings and Precautions (5.1)].
Clostridium difficile-Associated Diarrhea
Diarrhea is a common problem caused by antibacterial drugs, including Cefotetan for Injection and Dextrose Injection, which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
Drug Resistance
Patients should be counseled that antibacterial drugs, including Cefotetan for Injection and Dextrose Injection, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefotetan for Injection and Dextrose Injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefotetan for Injection and Dextrose Injection or other antibacterial drugs in the future.
Disulfiram-like Reaction
A disulfiram-like reaction characterized by flushing, sweating, headache, and tachycardia may occur when alcohol (beer, wine, etc.) is ingested within 72 hours after Cefotetan for Injection and Dextrose Injection administration. Patients should be cautioned about the ingestion of alcoholic beverages following the administration of Cefotetan for Injection and Dextrose Injection.
Rx only
DUPLEX is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Prepared in USA.
API from USA and Italy.
Y36-002-980 LD-198-4
Cefotetan for Injection USP
and Dextrose Injection USP
1g*
REF 3173-11
NDC 0264-3173-11
DUPLEX® CONTAINER
50 mL
Use only after mixing contents of both chambers.
For IV Use Only
Iso-osmotic
Single-Dose
Sterile/Nonpyrogenic
* Contains Cefotetan disodium equivalent to 1 g cefotetan.
Reconstitution: Hold container with set port in a downward direction and fold the diluent chamber just below the solution meniscus. To activate seal, squeeze folded diluent chamber until seal between diluent and drug chamber opens, releasing diluent into drug chamber. Agitate the reconstituted solution until the drug powder is completely dissolved. Fold the container a second time and squeeze until seal between drug chamber and set port opens.
After reconstitution each 50 mL single dose unit contains: Cefotetan for Injection (equivalent to 1 g cefotetan) with approx. 1.79 g (3.58% w/v) Hydrous Dextrose in Water for Injection USP. Sodium content is 80 mg (3.5 mEq) per gram of cefotetan. Approximate osmolality: 290 mOsmol/kg.
Prior to Reconstitution: Store at 20-25°C (68-77°F). Excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature.] Use only if container and seals are intact. Do not peel foil strip until ready for use. After foil strip removal, product must be used within 7 days, but not beyond the labeled expiration date. Protect from light after removal of foil strip.
After Reconstitution: Use only if prepared solution is clear and free from particulate matter. Use within 12 hours if stored at room temperature or within 5 days if stored under refrigeration. Do not use in a series connection. Do not introduce additives into this container. Prior to administration check for minute leaks by squeezing container firmly. If leaks are found, discard container and solution as sterility may be impaired. Do not freeze.
Not made with natural rubber latex, PVC or DEHP.
Rx only
Y37-002-565
LD-208-3
B. Braun Medical Inc.
Bethlehem, PA 18018-3524
Prepared in USA. API from USA and Italy.
EXP
LOT

PEEL HERE
Drug Chamber
Discard unit if foil strip is damaged. Peel foil strip only when
ready for use. Visually inspect drug prior to reconstitution.
See package insert for complete directions for reconstitution
and administration.
LD-336-1 X27-001-485

Cefotetan for Injection USP
and Dextrose Injection USP
2g*
REF 3175-11
NDC 0264-3175-11
DUPLEX® CONTAINER
50 mL
Use only after mixing contents of both chambers.
For IV Use Only
Iso-osmotic
Single-Dose
Sterile/Nonpyrogenic
* Contains Cefotetan disodium equivalent to 2 g cefotetan.
Reconstitution: Hold container with set port in a downward direction and fold the diluent chamber just below the solution meniscus. To activate seal, squeeze folded diluent chamber until seal between diluent and drug chamber opens, releasing diluent into drug chamber. Agitate the reconstituted solution until the drug powder is completely dissolved. Fold the container a second time and squeeze until seal between drug chamber and set port opens.
After reconstitution each 50 mL single dose unit contains: Cefotetan for Injection (equivalent to 2 g cefotetan) with approx. 1.04 g (2.08% w/v) Hydrous Dextrose in Water for Injection USP. Sodium content is 80 mg (3.5 mEq) per gram of cefotetan. Approximate osmolality: 290 mOsmol/kg.
Prior to Reconstitution: Store at 20-25°C (68-77°F). Excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature.] Use only if container and seals are intact. Do not peel foil strip until ready for use. After foil strip removal, product must be used within 7 days, but not beyond the labeled expiration date. Protect from light after removal of foil strip.
After Reconstitution: Use only if prepared solution is clear and free from particulate matter. Use within 12 hours if stored at room temperature or within 5 days if stored under refrigeration. Do not use in a series connection. Do not introduce additives into this container. Prior to administration check for minute leaks by squeezing container firmly. If leaks are found, discard container and solution as sterility may be impaired. Do not freeze.
Not made with natural rubber latex, PVC or DEHP.
Rx only
Y37-002-566
LD-209-3
B. Braun Medical Inc.
Bethlehem, PA 18018-3524
Prepared in USA. API from USA and Italy.
EXP
LOT

PEEL HERE
Drug Chamber
Discard unit if foil strip is damaged. Peel foil strip only when
ready for use. Visually inspect drug prior to reconstitution.
See package insert for complete directions for reconstitution
and administration.
LD-336-1 X27-001-485

| CEFOTETAN AND DEXTROSE
cefotetan and dextrose injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEFOTETAN AND DEXTROSE
cefotetan and dextrose injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - B. Braun Medical Inc. (002397347) |