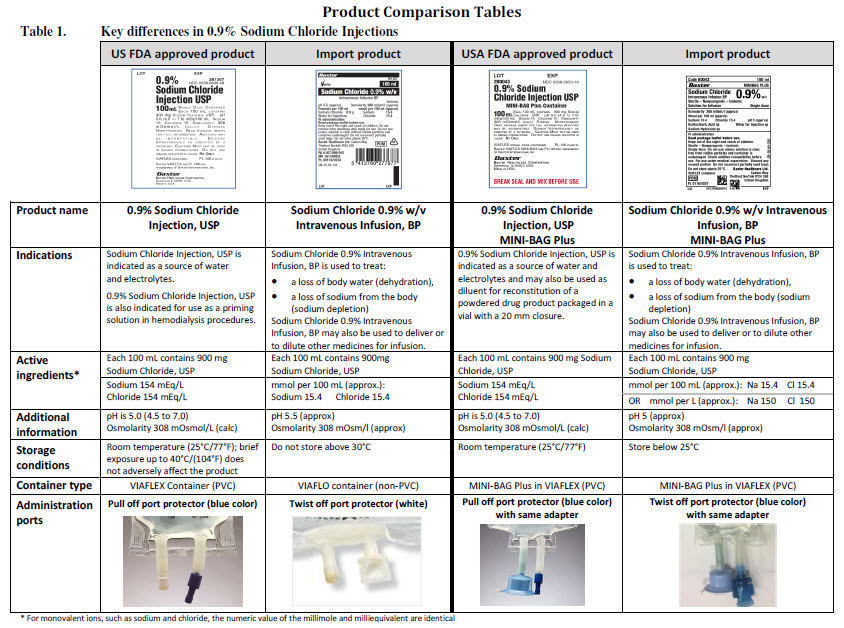
SODIUM CHLORIDE- sodium chloride injection
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sodium Chloride Intravenous Infusion BP 0.9% w/v
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Code B0042
50 ml
Baxter Logo
MINIBAG PLUS
Sodium Chloride 0.9% w/v
Intravenous Infusion BP
Sterile – Nonpyrogenic – Isotonic
Solution for Infusion Single dose
Osmolarity 308 m0sm/l (approx)
Mmol per 50ml (approx)
Sodium 7.7 Chloride 7.7 pH 5 (approx)
Hydrochloric Acid qs Water for Injection qs
Sodium Hydroxide qs
IV administration
Read package leaflet before use.
Keep out of the sight and reach of children.
Sterile – Nonpyrogenic – Isotonic
Single dose Do not use unless solution is clear,
free from visible particles and container is
undamaged Check additive compatibility before
use For use under medical supervision Discard any
unused portion Do not reconnect partially used bags
Do not store above 25°C
VIAFLEX container
POM symbol
PL0116/5057
Baxter Healthcare Ltd.
Caxton Way
Thetford Norfolk IP24 3SE
United Kingdom
Lot
5413760020907
EXP
CB-35-04-229
Code B0043
100 ml
Baxter Logo
MINIBAG PLUS
Sodium Chloride 0.9% w/v
Intravenous Infusion BP
Sterile – Nonpyrogenic – Isotonic
Solution for Infusion Single dose
Osmolarity 308 m0sm/l (approx)
Mmol per 50ml (approx)
Sodium 15.4 Chloride 15.4 pH 5 (approx)
Hydrochloric Acid qs Water for Injection qs
Sodium Hydroxide qs
IV administration
Read package leaflet before use.
Keep out of the sight and reach of children.
Sterile – Nonpyrogenic – Isotonic
Single dose Do not use unless solution is clear,
free from visible particles and container is
undamaged Check additive compatibility before
use For use under medical supervision Discard any
unused portion Do not reconnect partially used bags
Do not store above 25°C
VIAFLEX container
POM symbol
PL0116/5057
Baxter Healthcare Ltd.
Caxton Way
Thetford Norfolk IP24 3SE
United Kingdom
Lot
5413760020914
EXP
CB-35-04-230
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare S.A. | 988899845 | ANALYSIS(0338-9616) , LABEL(0338-9616) , MANUFACTURE(0338-9616) , PACK(0338-9616) , STERILIZE(0338-9616) | |