Label: AMRIX- cyclobenzaprine hydrochloride capsule, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 16590-596-10, 16590-596-15, 16590-596-28, 16590-596-30, view more16590-596-60, 16590-596-90, 16590-810-30, 16590-810-60, 16590-810-90 - Packager: STAT RX USA LLC
- This is a repackaged label.
- Source NDC Code(s): 63459-700, 63459-701
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 18, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
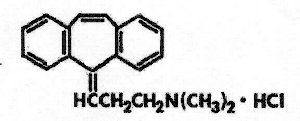
AMRIX® (Cyclobenzaprine Hydrochloride Extended-Release Capsules) is a skeletal muscle relaxant which relieves muscle spasm of local origin without interfering with muscle function. The active ingredient in AMRIX extended-release capsules is cyclobenzaprine hydrochloride, USP. Cyclobenzaprine hydrochloride (HCl) is a white, crystalline tricyclic amine salt with the empirical formula C20H21N•HCl and a molecular weight of 311.9. It has a melting point of 217°C, and a pKa of 8.47 at 25°C. It is freely soluble in water and alcohol, sparingly soluble in isopropanol, and insoluble in hydrocarbon solvents. If aqueous solutions are made alkaline, the free base separates. Cyclobenzaprine HCl is designated chemically as 3-(5H-dibenzo[a,d] cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride, and has the following structural formula:
AMRIX extended-release capsules for oral administration are supplied in 15 and 30 mg strengths. AMRIX capsules contain the following inactive ingredients: diethyl phthalate NF, ethylcellulose NF (Ethocel Standard 10 Premium), gelatin, Opadry® Clear YS-1-7006, sugar spheres NF (20-25 mesh), and titanium dioxide. AMRIX 15 mg capsules also contain D&C yellow #10, FD&C green #3, and FD&C red #40. AMRIX 30 mg capsules also contain FD&C blue #1, FD&C blue #2, FD&C red #40, and FD&C yellow #6.
-
CLINICAL PHARMACOLOGY
Cyclobenzaprine relieves skeletal muscle spasm of local origin without interfering with muscle function. Cyclobenzaprine has not been shown to be effective in muscle spasm due to central nervous system disease. In animal models, cyclobenzaprine reduced or abolished skeletal muscle hyperactivity. Animal studies indicate that cyclobenzaprine does not act at the neuromuscular junction or directly on skeletal muscle. Such studies show that cyclobenzaprine acts primarily within the central nervous system at the brain stem as opposed to the spinal cord level, although an overlapping action on the latter may contribute to its overall skeletal muscle relaxant activity. Evidence suggests that the net effect of cyclobenzaprine is a reduction of tonic somatic motor activity, influencing both gamma (γ) and alpha (α) motor systems. Pharmacological studies in animals demonstrated a similarity between the effects of cyclobenzaprine and the structurally related tricyclic antidepressants, including reserpine antagonism, norepinephrine potentiation, potent peripheral and central anticholinergic effects, and sedation. Cyclobenzaprine caused slight to moderate increase in heart rate in animals.
PharmacokineticsAbsorptionIn a single-dose study comprised of healthy adult males (n=15), the dose adjusted ratios of the arithmetic means of AUC0-168 and AUC0-∞ indicated that exposure of the AMRIX 30 mg was about 16% and 10% higher than that of AMRIX 15 mg, respectively. The dose-adjusted ratios of the arithmetic means of Cmax indicated that the peak plasma concentration of AMRIX 30 mg was about 20% higher than that of AMRIX 15 mg. The half-lives and time to peak plasma cyclobenzaprine concentration were similar for both AMRIX 15 mg and 30 mg. These data are summarized below.
Table 1: Summary of Pharmacokinetic Parameters in Healthy Adult Subjects Parameter
Mean ± SDAMRIX 15 mg
(N=15)AMRIX 30 mg
(N=14)AUC0-168 (ng•hr/mL) 318.3 ± 114.7 736.6 ± 259.4 AUC0-∞ (ng•hr/mL) 354.1 ± 119.8 779.9 ± 277.6 Cmax (ng/mL) 8.3 ± 2.2 19.9 ± 5.9 Tmax (hrs) 8.1 ± 2.9 7.1 ± 1.6 t1/2 (hrs) 33.4 ± 10.3 32.0 ± 10.1 SD = standard deviation
A food effect study conducted in healthy adult subjects (n=15) utilizing a single dose of AMRIX 30 mg demonstrated a statistically significant increase in bioavailability when AMRIX 30 mg was given with food relative to the fasted state. There was a 35% increase in peak plasma cyclobenzaprine concentration (Cmax) and a 20% increase in exposure (AUC0-168 and AUC0-∞) in the presence of food. No effect, however, was noted in Tlag, Tmax, or the shape of the mean plasma cyclobenzaprine concentration versus time profile. Cyclobenzaprine in plasma was first detectable in both the fed and fasted states at 1.5 hours.
In a multiple-dose study utilizing AMRIX 30 mg administered once daily for 7 days in a group of healthy adult volunteers (n=35) a 2.5-fold accumulation of plasma cyclobenzaprine levels was noted at steady-state.
Metabolism and EliminationCyclobenzaprine is extensively metabolized and is excreted primarily as glucuronides via the kidney. Cytochromes P-450 3A4, 1A2, and, to a lesser extent, 2D6, mediate N-demethylation, one of the oxidative pathways for cyclobenzaprine. Cyclobenzaprine has an elimination half-life of 32 hours (range 8-37 hours; n=18); plasma clearance is 0.7 L/min following single dose administration of AMRIX.
Special PopulationsElderlyAlthough there were no notable differences in Cmax or Tmax, cyclobenzaprine plasma AUC is increased by 40% and the plasma half-life of cyclobenzaprine is prolonged in elderly subjects greater than 65 years of age (50 hours) after dosing with AMRIX compared to younger subjects (32 hours). Pharmacokinetic characteristics of cyclobenzaprine following multiple-dose administration of AMRIX in the elderly were not evaluated.
Table 2: Summary of Pharmacokinetic Parameters of AMRIX 30 mg Extended-Release Capsules, By Age Group Parameter Mean ± SD AMRIX 30 mg QD 18 to 45 years
(N=18)65 to 75 years
(N=17)AUC0-168 (ng•hr/mL) 715.1 ± 264.2 945.9 ± 255.2 AUC0-∞ (ng•hr/mL) 751.2 ± 271.5 1055.2 ± 301.9 Cmax (ng/mL)* 19.2 ± 5.6 19.2 ± 5.1 Tmax (hrs)* 6.8 ± 1.9 8.5 ± 2.3 t1/2 (hrs) 32.4 ± 8.1 49.0 ± 8.3 * Measured over the entire 24 hour period
SD= standard deviation
Hepatic ImpairmentIn a pharmacokinetic study of immediate-release cyclobenzaprine in 16 subjects with hepatic impairment (15 mild, 1 moderate per Child-Pugh score), both AUC and Cmax were approximately double the values seen in the healthy control group. The pharmacokinetics of cyclobenzaprine in subjects with severe hepatic impairment is not known.
-
CLINICAL STUDIES
Efficacy was assessed in two double-blind, parallel-group, placebo-controlled studies of identical design of AMRIX 15 mg and 30 mg taken once daily in patients with muscle spasms associated with acute painful musculoskeletal conditions.
There were significant differences in the primary efficacy analysis, the patient’s rating of medication helpfulness, between the AMRIX 15 mg group and the placebo group at Days 4 and 14 in one study and between the AMRIX 30 mg group and the placebo group at Day 4 in the second study.
Table 3: Subject’s Rating of Medication Helpfulness - Study 1105
Day 4 Day 14
Number of Subjects (%) Number of Subjects (%)
Placebo
(N = 64)AMRIX 30 mg
(N = 64)Placebo
(N = 64)AMRIX 30 mg
(N = 64)Excellent 1 (1.6%) 3 (4.7%) 12 (18.8%) 15 (23.4%) Very Good 5 (7.8%) 13 (20.3%) 9 (14.1%) 19 (29.7%) Good 15 (23.4%) 22 (34.4%) 10 (15.6%) 15 (23.4%) Fair 24 (37.5%) 20 (31.3%) 16 (25.0%) 10 (15.6%) Poor 10 (15.6%) 5 (7.8%) 9 (14.1%) 4 (6.3%) Missing 9 (14.1%) 1 (1.6%) 8 (12.5%) 1 (1.6%)
Table 4: Subject's Rating of Medication Helpfulness - Study 1106
Day 4 Day 14
Number of Subjects (%) Number of Subjects (%)
Placebo
(N = 64)AMRIX 15 mg
(N = 63)Placebo
(N = 64)AMRIX 15 mg
(N = 63)Excellent 1 (1.6%) 2 (3.2%) 10 (15.6%) 13 (20.6%) Very Good 10 (15.6%) 12 (19.0%) 12 (18.8%) 21 (33.3%) Good 14 (21.9%) 21 (33.3%) 13 (20.3%) 9 (14.3%) Fair 16 (25.0%) 17 (27.0%) 14 (21.9%) 10 (15.9%) Poor 19 (29.7%) 6 (9.5%) 12 (18.8%) 5 (7.9%) Missing 4 (6.3%) 5 (17.9%) 3 (4.7%) 5 (7.9%) In addition, one of the two studies demonstrated significant differences between the AMRIX 30 mg group and the placebo group in terms of patient-rated relief from local pain due to muscle spasm at Day 4 and Day 8, in subject-rated restriction of movement at Day 4 and Day 8, and in patient-rated global impression of change at Day 4, Day 8, and Day 14.
There were no significant treatment differences between the AMRIX treatment groups and the placebo group in physician's global assessment, in subject-rated restriction in activities of daily living, or quality of night-time sleep.
-
INDICATIONS AND USAGE
AMRIX is indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, and limitation of motion.
AMRIX should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted.
AMRIX has not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease or in children with cerebral palsy.
-
CONTRAINDICATIONS
- Hypersensitivity to any component of this product.
- Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation.
- Hyperpyretic crisis seizures and deaths have occurred in patients receiving cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitor drugs.
- During the acute recovery phase of myocardial infarction, and in patients with arrhythmias, heart block conduction disturbances, or congestive heart failure.
- Hyperthyroidism.
-
WARNINGS
AMRIX is closely related to the tricyclic antidepressants, e.g., amitriptyline and imipramine. In short term studies for indications other than muscle spasm associated with acute musculoskeletal conditions, and usually at doses somewhat greater than those recommended for skeletal muscle spasm, some of the more serious central nervous system reactions noted with the tricyclic antidepressants have occurred (see WARNINGS, below, and ADVERSE REACTIONS).
Tricyclic antidepressants have been reported to produce arrhythmias, sinus tachycardia, prolongation of the conduction time leading to myocardial infarction and stroke. AMRIX may enhance the effects of alcohol, barbiturates, and other CNS depressants.
As a result of a two-fold higher cyclobenzaprine plasma levels in subjects with mild hepatic impairment, as compared to healthy subjects, following administration of immediate-release cyclobenzaprine and because there is limited dosing flexibility with AMRIX, use of AMRIX is not recommended in subjects with mild, moderate or severe hepatic impairment.
As a result of a 40% increase in cyclobenzaprine plasma levels and a 56% increase in plasma half-life following administration of AMRIX in elderly subjects as compared to young adults, use of AMRIX is not recommended in elderly.
-
PRECAUTIONS
General
Because of its atropine-like action, AMRIX should be used with caution in patients with a history of urinary retention, angle-closure glaucoma, increased intraocular pressure, and in patients taking anticholinergic medication.
Information for PatientsAMRIX, especially when used with alcohol or other CNS depressants, may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle.
Drug InteractionsAMRIX may have life-threatening interactions with MAO inhibitors. (See CONTRAINDICATIONS.) AMRIX may enhance the effects of alcohol, barbiturates, and other CNS depressants. Tricyclic antidepressants may block the antihypertensive action of guanethidine and similarly acting compounds. Tricyclic antidepressants may enhance the seizure risk in patients taking tramadol (ULTRAM® [tramadol HCl tablets, Ortho-McNeil Pharmaceutical] or ULTRACET® [tramadol HCl and acetaminophen tablets, Ortho-McNeil Pharmaceutical]).
Carcinogenesis, Mutagenesis, Impairment of FertilityIn rats treated with cyclobenzaprine for up to 67 weeks at doses of approximately 5 to 40 times the maximum recommended human dose, pale, sometimes enlarged, livers were noted and there was a dose-related hepatocyte vacuolation with lipidosis. In the higher dose groups, this microscopic change was seen after 26 weeks and even earlier in rats that died prior to 26 weeks; at lower doses, the change was not seen until after 26 weeks. Cyclobenzaprine did not affect the onset, incidence, or distribution of neoplasia in an 81-week study in the mouse or in a 105-week study in the rat. At oral doses of up to 10 times the human dose, cyclobenzaprine did not adversely affect the reproductive performance or fertility of male or female rats. Cyclobenzaprine did not demonstrate mutagenic activity in the male mouse at dose levels of up to 20 times the human dose.
A battery of mutagenicity tests using bacterial and mammalian systems for point mutations and cytogenic effects have provided no evidence for a mutagenic potential for cyclobenzaprine. An in vivo mouse bone micronucleus assay, an assessment of chromosomal aberrations (Chinese hamster ovary), and a mammalian microsome reverse mutation assay were negative.
PregnancyPregnancy Category B: Reproduction studies have been performed in rats, mice, and rabbits at doses up to 20 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to cyclobenzaprine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing MothersIt is not known whether this drug is excreted in human milk. Because cyclobenzaprine is closely related to the tricyclic antidepressants, some of which are known to be excreted in human milk, caution should be exercised when AMRIX is administered to a nursing woman.
Pediatric UseSafety and effectiveness of AMRIX has not been studied in pediatric patients.
Use in the ElderlyThe plasma concentration and half-life of cyclobenzaprine are substantially increased in the elderly when compared to the general patient population (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations, Elderly). Accordingly, AMRIX should not be used in the elderly.
-
ADVERSE REACTIONS
The most common adverse reactions in the two 14-day clinical efficacy trials and in the 7-day repeat-dose pharmacokinetic study are presented in Tables 5 and 6, respectively.
Table 5: Incidence of the Most Common Adverse Reactions Occurring in ≥ 3% of Subjects in Any Treatment Group in the Two Phase 3, Double-Blind AMRIX Trials
AMRIX 15 mg
N=127AMRIX 30 mg
N=126Placebo
N=128Dry mouth 6% 14% 2% Dizziness 3% 6% 2% Fatigue 3% 3% 2% Constipation 1% 3% 0% Somnolence 1% 2% 0% Nausea 3% 3% 1% Dyspepsia 0% 4% 1% Table 6: Incidence of the Most Common Adverse Reactions Occurring in ≥ 3% of Subjects in Any Treatment Group in the Seven-Day Pharmacokinetic Study of AMRIX
AMRIX 30 mg
N = 36Somnolence 100% Dry mouth 58% Headache NOS 17% Dizziness 19% Vision blurred 3% Nausea 8% Dysgeusia 6% Palpitations 6% Tremor 6% Dry throat 8% Acne NOS 6% Disturbance in attention 6% Insomnia 0 In a postmarketing surveillance program (7607 patients treated with cyclobenzaprine 10 mg TID), the adverse reactions reported most frequently were drowsiness, dry mouth, and dizziness. The incidence of these common adverse reactions was lower in the surveillance program than in the controlled clinical studies:
Table 7: Most Common Adverse Reactions from Postmarketing Surveillance Program
Clinical Studies
cyclobenzaprine 10 mg TIDSurveillance Program
cyclobenzaprine 10 mg TIDDrowsiness 39% 16% Dry mouth 27% 7% Dizziness 11% 3% Among the less frequent adverse reactions, there was no appreciable difference in incidence in controlled clinical studies or in the surveillance program. Adverse reactions which were reported in 1% to 3% of the patients were: fatigue/tiredness, asthenia, nausea, constipation, dyspepsia, unpleasant taste, blurred vision, headache, nervousness, and confusion. The following adverse reactions have been reported in post-marketing experience or with an incidence of less than 1% of patients in clinical trials with the 10 mg TID tablet:
Body as a Whole: Syncope; malaise.
Cardiovascular: Tachycardia; arrhythmia; vasodilatation; palpitation; hypotension.
Digestive: Vomiting; anorexia; diarrhea; gastrointestinal pain; gastritis; thirst; flatulence; edema of the tongue; abnormal liver function and rare reports of hepatitis, jaundice, and cholestasis.
Hypersensitivity: Anaphylaxis; angioedema; pruritus; facial edema; urticaria; rash.
Musculoskeletal: Local weakness.
Nervous System and Psychiatric: Seizures, ataxia; vertigo; dysarthria; tremors; hypertonia; convulsions; muscle twitching; disorientation; insomnia; depressed mood; abnormal sensations; anxiety; agitation; psychosis, abnormal thinking and dreaming; hallucinations; excitement; paresthesia; diplopia.
Skin: Sweating.
Special Senses: Ageusia; tinnitus.
Urogenital: Urinary frequency and/or retention.Causal Relationship Unknown
Other reactions, reported rarely for cyclobenzaprine under circumstances where a causal relationship could not be established or reported for other tricyclic drugs, are listed to serve as alerting information to physicians:
Body as a Whole: Chest pain; edema.
Cardiovascular: Hypertension; myocardial infarction; heart block; stroke.
Digestive: Paralytic ileus, tongue discoloration; stomatitis; parotid swelling.
Endocrine: Inappropriate ADH syndrome.
Hematic and Lymphatic: Purpura; bone marrow depression; leukopenia; eosinophilia; thrombocytopenia.
Metabolic, Nutritional and Immune: Elevation and lowering of blood sugar levels; weight gain or loss.
Musculoskeletal: Myalgia.
Nervous System and Psychiatric: Decreased or increased libido; abnormal gait; delusions; aggressive behavior; paranoia; peripheral neuropathy; Bell’s palsy; alteration in EEG patterns; extrapyramidal symptoms.
Respiratory: Dyspnea.
Skin: Photosensitization; alopecia.
Urogenital: Impaired urination; dilatation of urinary tract; impotence; testicular swelling; gynecomastia; breast enlargement; galactorrhea. -
DRUG ABUSE AND DEPENDENCE
Pharmacologic similarities among the tricyclic drugs require that certain withdrawal symptoms be considered when AMRIX is administered, even though they have not been reported to occur with this drug. Abrupt cessation of treatment after prolonged administration rarely may produce nausea, headache, and malaise. These are not indicative of addiction.
-
OVERDOSAGE
Although rare, deaths may occur from overdosage with AMRIX. Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose. As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity may develop rapidly after cyclobenzaprine overdose; therefore, hospital monitoring is required as soon as possible. The acute oral LD50 of cyclobenzaprine is approximately 338 and 425 mg/kg in mice and rats, respectively.
ManifestationsThe most common effects associated with cyclobenzaprine overdose are drowsiness and tachycardia. Less frequent manifestations include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations. Rare but potentially critical manifestations of overdose are cardiac arrest, chest pain, cardiac dysrhythmias, severe hypotension, seizures, and neuroleptic malignant syndrome. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of cyclobenzaprine toxicity. Other potential effects of overdosage include any of the symptoms listed under ADVERSE REACTIONS.
ManagementGeneralAs management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment.
In order to protect against the rare but potentially critical manifestations described above, obtain an ECG and immediately initiate cardiac monitoring. Protect the patient’s airway, establish an intravenous line, and initiate gastric decontamination. Observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. Monitoring of plasma drug levels should not guide management of the patient. Dialysis is probably of no value because of low plasma concentrations of the drug.
Gastrointestinal DecontaminationAll patients suspected of an overdose with AMRIX should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage and emesis is contraindicated.
CardiovascularA maximal limb-lead QRS duration of 0.10 seconds may be the best indication of the severity of the overdose. Serum alkalinization, to a pH of 7.45 to 7.55, using intravenous sodium bicarbonate and hyperventilation (as needed), should be instituted for patients with dysrhythmias and/or QRS widening. A pH >7.60 or a pCO2 <20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium, or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
CNSIn patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines or, if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in close consultation with a poison control center.
Psychiatric Follow-upSince overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric ManagementThe principles of management of child and adult overdosage are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
-
DOSAGE AND ADMINISTRATION
The recommended adult dose for most patients is one (1) AMRIX 15 mg capsule taken once daily. Some patients may require up to 30 mg/day, given as one (1) AMRIX 30 mg capsule taken once daily or as two (2) AMRIX 15 mg capsules taken once daily.
It is recommended that doses be taken at approximately the same time each day.
Use of AMRIX for periods longer than two or three weeks is not recommended (see INDICATIONS AND USAGE).
Dosage Considerations for Special Patient Populations: AMRIX should not be used in the elderly or in patients with impaired hepatic function. (see WARNINGS)
-
HOW SUPPLIED
AMRIX extended-release capsules are available in 15 and 30 mg strengths, packaged in bottles of 60 capsules. AMRIX 15 mg capsules (NDC 63459-700-60) are orange/orange and are embossed in blue ink with “15 mg” on the body, and Cephalon “C” logo, “Cephalon”, and a dashed band on the cap. AMRIX 30 mg capsules (NDC 63459-701-60) are blue/orange and are embossed in white ink with “30 mg” on the body, and Cephalon “C” logo, “Cephalon”, and a dashed band on the cap.
Dispense in a tight, light-resistant container as defined in the USP/NF.
Store at 25°C (77°F); excursions permitted to 15 - 30°C (59 - 86°F); [see USP Controlled Room Temperature].KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Distributed by:
Cephalon, Inc.
Frazer, PA 19355Manufactured by:
Eurand Inc.
Vandalia, Ohio 45377
AMRIX is a trademark of Cephalon, Inc. or its affiliates.U.S. Patent No. 7,387,793
© 2004, 2006, 2007, 2008 Cephalon, Inc., or its affiliates. All rights reserved.
PI-40018-01
Revised December 2008
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMRIX
cyclobenzaprine hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16590-596(NDC:63459-700) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOBENZAPRINE HYDROCHLORIDE (UNII: 0VE05JYS2P) (CYCLOBENZAPRINE - UNII:69O5WQQ5TI) CYCLOBENZAPRINE 15 mg Inactive Ingredients Ingredient Name Strength DIETHYL PHTHALATE (UNII: UF064M00AF) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) GELATIN (UNII: 2G86QN327L) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C RED NO. 40 (UNII: WZB9127XOA) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color orange Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 15mg;Cephalon Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16590-596-10 10 in 1 BOTTLE 2 NDC:16590-596-15 15 in 1 BOTTLE 3 NDC:16590-596-28 28 in 1 BOTTLE 4 NDC:16590-596-30 30 in 1 BOTTLE 5 NDC:16590-596-60 60 in 1 BOTTLE 6 NDC:16590-596-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021777 10/01/2007 AMRIX
cyclobenzaprine hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16590-810(NDC:63459-701) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOBENZAPRINE HYDROCHLORIDE (UNII: 0VE05JYS2P) (CYCLOBENZAPRINE - UNII:69O5WQQ5TI) CYCLOBENZAPRINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength DIETHYL PHTHALATE (UNII: UF064M00AF) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) GELATIN (UNII: 2G86QN327L) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SUCROSE (UNII: C151H8M554) Product Characteristics Color blue, orange Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 30mg;Cephalon Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16590-810-30 30 in 1 BOTTLE 2 NDC:16590-810-60 60 in 1 BOTTLE 3 NDC:16590-810-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021777 10/01/2007 Labeler - STAT RX USA LLC (786036330) Establishment Name Address ID/FEI Business Operations STAT RX USA LLC 786036330 relabel, repack




