Label: SODIUM CHLORIDE irrigant
- NDC Code(s): 0338-0048-02, 0338-0048-03, 0338-0048-04, 0338-0048-05
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 21, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- INDICATIONS AND USAGE
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
-
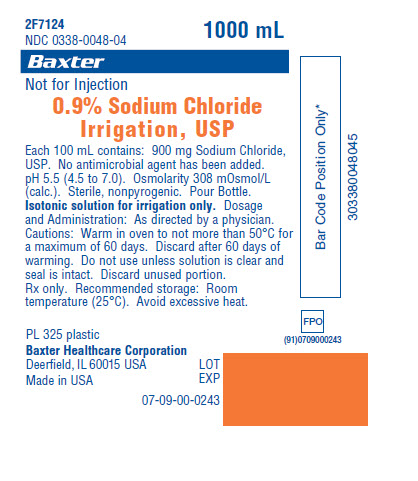
PRINCIPAL DISPLAY PANEL - PACKAGING LABELING
Container Label
2F7124
NDC 0338-0048-041000 mL
Baxter Logo
Not for Injection
0.9% Sodium Chloride Irrigation, USP
Each 100 mL contains: 900 mg Sodium
Chloride, USP. No antimicrobial agent has
been added. pH 5.5 (4.5 to 7.0). Osmolarity
308 mOsmol/L (calc.). Sterile, nonpyrogenic.
Pour Bottle. Isotonic solution for irrigation
only. Dosage and Administration: As
directed by a physician. Cautions: Warm in
oven to not more than 50°C for a maximum
of 60 days. Discard after 60 days of
warming. Do not use unless solution is clear
and seal is intact. Discard unused portion.
Rx only. Recommended storage: Room
temperature (25°C). Avoid excessive heat.PL 325 plastic
Baxter Healthcare Corporation
Deerfield, IL 60015 USAMade in USA
LOT
EXP07-09-00-0243
Bar Code Position Only*
303380048045FPO
(91)0709000243 -
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0048 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0048-02 24 in 1 CARTON 12/31/1974 1 250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:0338-0048-03 18 in 1 CARTON 12/31/1974 2 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:0338-0048-04 12 in 1 CARTON 12/31/1974 3 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC:0338-0048-05 9 in 1 CARTON 12/31/1974 10/31/2022 4 1500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017427 12/31/1974 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 001728059 ANALYSIS(0338-0048) , MANUFACTURE(0338-0048) , LABEL(0338-0048) , PACK(0338-0048) , STERILIZE(0338-0048) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0048)