MANNITOL- mannitol injection, solution
Hospira, Inc.
----------
Mannitol Intravenous
Mannitol Injection, USP
Flexible Plastic Container
DESCRIPTION
Mannitol Intravenous (Mannitol Injection, USP) is a sterile, nonpyrogenic solution of mannitol in water for injection available in a concentration of 20% in flexible plastic containers.
The content and characteristics of the available concentration is as follows:
| Conc. (%) | g/100 mL | mOsmol/liter (calc.) | pH* |
|---|---|---|---|
|
|||
| 20 | 20 | 1098 | 6.3 (4.5 to 7.0) |
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Mannitol Injection, USP is a parenteral obligatory osmotic diuretic.
Mannitol, USP is chemically designated D-mannitol (C6H14O6), a white crystalline powder or free-flowing granules freely soluble in water. It has the following structural formula:
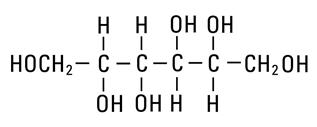
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap, but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
CLINICAL PHARMACOLOGY
When administered intravenously mannitol is confined to the extracellular space, only slightly metabolized and rapidly excreted by the kidney. Approximately 80% of a 100 g dose appears in the urine in 3 hours. The drug is freely filtered by the glomeruli with less than 10% tubular reabsorption; it is not secreted by tubular cells. Mannitol induces diuresis by elevating the osmolarity of the glomerular filtrate and thereby hindering tubular reabsorption of water. Excretion of sodium and chloride is also enhanced.
INDICATIONS AND USAGE
Mannitol Intravenous (Mannitol Injection, USP) is indicated for the following purposes in adults and pediatric patients.
CONTRAINDICATIONS
- Well established anuria due to severe renal disease.
- Severe pulmonary congestion or frank pulmonary edema.
- Active intracranial bleeding except during craniotomy.
- Severe dehydration.
- Progressive heart failure or pulmonary congestion after institution of mannitol therapy.
- Do not administer to patients with a known hypersensitivity to mannitol.
WARNINGS
- Renal complications, including irreversible renal failure have been reported in patients receiving mannitol. Reversible, oliguric acute kidney injury (AKI) has occurred in patients with normal pretreatment renal function who received mannitol. Patients with pre-existing renal disease, patients with conditions that put them at risk for renal failure, or those receiving potentially nephrotoxic drugs or other diuretics, are at increased risk of renal failure. Avoid concomitant administration of nephrotoxic drugs (e.g., aminoglycosides) or other diuretics with mannitol (See PRECAUTIONS). If urine output declines during mannitol infusion, the patient's clinical status should be closely reviewed and mannitol infusion suspended if necessary.
- The obligatory diuretic response following rapid infusion of 20% mannitol may further aggravate pre-existing hemoconcentration. Excessive loss of water and electrolytes may lead to serious imbalances. Serum sodium and potassium should be carefully monitored during mannitol administration.
- Accumulation of mannitol may result in overexpansion of the extracellular fluid which may intensify existing or latent congestive heart failure.
- Excessive loss of water and electrolytes may lead to serious imbalances. With continued administration of mannitol, loss of water in excess of electrolytes can cause hypernatremia. Electrolyte measurements, including sodium and potassium are therefore of vital importance in monitoring the infusion of mannitol.
- Osmotic nephrosis, a reversible vacuolization of the tubules of no known clinical significance, may proceed to severe irreversible nephrosis, so that the renal function must be closely monitored during mannitol infusion.
- Mannitol injection may increase cerebral blood flow and the risk of postoperative bleeding in neurosurgical patients.
- For intravenous use only. Do not administer intramuscularly or subcutaneously. Never add mannitol in whole blood for transfusion.
- Mannitol may increase the cerebral blood flow and worsen intracranial hypertension in children who develop a generalized cerebral hyperemia during the first 24 to 48 hours post injury.
PRECAUTIONS
- The cardiovascular status of the patient should be carefully evaluated before rapidly administering mannitol since sudden expansion of the extracellular fluid may lead to fulminating congestive heart failure.
- Shift of sodium-free intracellular fluid into the extracellular compartment following mannitol infusion may lower serum sodium concentration and aggravate pre-existing hyponatremia.
- By sustaining diuresis, mannitol administration may obscure and intensify inadequate hydration or hypovolemia.
- Avoid concomitant administration of nephrotoxic drugs (e.g., aminoglycosides) or other diuretics with mannitol.
- Electrolyte-free mannitol solutions should not be given conjointly with blood. If it is essential that blood be given simultaneously, at least 20 mEq of sodium chloride should be added to each liter of mannitol solution to avoid pseudoagglutination.
- When exposed to low temperatures, solutions of mannitol may crystallize. If crystals are observed, the container should be warmed to redissolve, then cooled to body temperature before administering. (See NOTE under HOW SUPPLIED.) When infusing 20% mannitol, the administration set should include a filter. Do not infuse mannitol solution if crystals are present.
- Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with solutions from flexible plastic containers have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy
Animal reproduction studies have not been conducted with mannitol injection. It is also not known whether mannitol injection can cause fetal harm when given to a pregnant woman or can affect reproduction. Mannitol injection should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when mannitol is administered to a nursing woman.
Pediatric Use
(See DOSAGE AND ADMINISTRATION sections.) Safety and effectiveness of solutions from flexible plastic containers in pediatric patients have not been well established.
ADVERSE REACTIONS
Adverse reactions more commonly reported during or after the infusion of mannitol include: Pulmonary congestion, fluid and electrolyte imbalance, acidosis, electrolyte loss, dryness of mouth, thirst, marked diuresis, urinary retention, edema, headache, blurred vision, convulsions, nausea, vomiting, rhinitis, arm pain, skin necrosis, thrombophlebitis, chills, dizziness, urticaria, dehydration, hypotension, tachycardia, fever and angina-like chest pains.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
OVERDOSAGE
Too rapid infusion of large amounts of mannitol will cause a shift of intracellular water into the extracellular compartment resulting in cellular dehydration and overexpansion of the intravascular space with hyponatremia, congestive heart failure and pulmonary edema. Repeated doses should not be given to patients with persistent oliguria as this can produce a hyperosmolar state and precipitate congestive heart failure and pulmonary edema due to volume overload. Dosage must be carefully monitored and adjusted in accordance with the clinical situation to avoid the consequences of overdosage. (See CONTRAINDICATIONS, WARNINGS, PRECAUTIONS and DOSAGE AND ADMINISTRATION).
DOSAGE AND ADMINISTRATION
Reduction of Intracranial Pressure and Brain Mass
In adults a dose of 0.25 to 2 g/kg body weight as a 20% solution administered over a period of 30 to 60 minutes; pediatric patients 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes. In small or debilitated patients, a dose of 500 mg/kg may be sufficient. Careful evaluation must be made of the circulatory and renal reserve prior to and during administration of mannitol at the higher doses and rapid infusion rates. Careful attention must be paid to fluid and electrolyte balance, body weight, and total input and output before and after infusion of mannitol. Evidence of reduced cerebral spinal fluid pressure must be observed within 15 minutes after starting infusion.
Reduction of Intraocular Pressure
In adults a dose of 0.25 to 2 g/kg body weight as a 20% solution administered over a period of 30 to 60 minutes; pediatric patients 1 to 2 g/kg body weight or 30 to 60 g/m2 body surface area over a period of 30 to 60 minutes. In small or debilitated patients, a dose of 500 mg/kg may be sufficient. When used preoperatively, the dose should be given one to one and one-half hours before surgery to achieve maximal reduction of intraocular pressure before operation.
Measurement of Glomerular Filtration Rate (GFR)
100 mL of a 20% solution (20 g) should be diluted with 180 mL of sodium chloride injection (normal saline) or 200 mL of a 10% solution (20 g) should be diluted with 80 mL of sodium chloride injection (normal saline). The resulting 280 mL of 7.2% solution is infused at a rate of 20 mL per minute. The urine is collected by catheter for a specific period of time and analyzed for mannitol excreted in mg per minute. A blood sample is drawn at the start and at the end of the time period and the concentration of mannitol determined in mg/mL of plasma. GFR is the number of mL of plasma that must have been filtered to account for the amount excreted per minute in the urine. Normal clearance rates are approximately 125 mL/minute for men; 116 mL/minute for women.
Drug Interactions
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives to the flexible container, use aseptic technique, mix thoroughly and do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration; whenever container and solution permit. (See PRECAUTIONS).
INSTRUCTIONS FOR USE
To Open
Tear outer wrap at notch and remove solution container. If supplemental medication is desired, follow directions below before preparing for administration. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Add Medication
- Prepare additive port.
- Using aseptic technique and an additive delivery needle of appropriate length, puncture resealable additive port at target area, inner diaphragm and inject. Withdraw needle after injecting medication.
- The additive port may be protected by covering with an additive cap.
- Mix container contents thoroughly.
Preparation for Administration
(Use aseptic technique)
- Close flow control clamp of administration set.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: See full directions on administration set carton.
- Suspend container from hanger.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- Open flow control clamp and clear air from set. Close clamp.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
HOW SUPPLIED
Mannitol Intravenous (Mannitol Injection, USP) is supplied in single-dose containers as follows:
| Unit of Sale | Concentration | Each |
|---|---|---|
| NDC 0409-7715-03
Case containing 12 | 100 g/500 mL (200 mg/mL) | NDC 0409-7715-13 500 mL Flexible Container |
| NDC 0409-7715-02
Case containing 24 | 50 g/250 mL (200 mg/mL) | NDC 0409-7715-12 250 mL Flexible Container |
NOTE: Crystals may form in mannitol solutions especially if the solutions are chilled. To dissolve crystals in the flexible container, warm the unit to 70°C with agitation. Heat solution by using a dry-heat cabinet with overwrap intact. The use of a water bath is not recommended. Cool to body temperature or less before administering. When infusing 20% mannitol, the administration set should include a filter.
PRINCIPAL DISPLAY PANEL - 75 g/500 mL Bag Label
500 mL
NDC 0409-7714-13
15% MANNITOL I.V.
MANNITOL INJECTION, USP
75 g/500 mL (150 mg/mL)
EACH 100 mL CONTAINS MANNITOL 15 GRAMS. MAY
CONTAIN SODIUM BICARBONATE FOR pH ADJUSTMENT.
823 mOsmol/LITER (CALC.)
STERILE, NONPYROGENIC.
pH 6.3 (4.5 to 7.0)
ADDITIVES MAY BE INCOMPATIBLE. CONSULT
WITH PHARMACIST, IF AVAILABLE.
WHEN INTRODUCING ADDITIVES, USE ASEPTIC
TECHNIQUE, MIX THOROUGHLY AND DO NOT STORE.
SINGLE-DOSE CONTAINER. FOR INTRAVENOUS USE.
USUAL DOSAGE: SEE INSERT. WARNING: SEE INSERT
REGARDING CONTRAINDICATION IN SEVERE RENAL
IMPAIRMENT. WARNING: IF CRYSTALS FORM, WARM
WITH OVERWRAP INTACT AND AGITATE TO DISSOLVE.
SEE INSERT. ADMINISTER INTRAVENOUSLY AT 30° TO
37°C (86° TO 98.6°F). INSPECT CAREFULLY. DISCARD
UNUSED PORTION. USE ONLY IF SOLUTION IS CLEAR AND
CONTAINER IS UNDAMAGED. MUST NOT BE USED IN
SERIES CONNECTIONS.
Rx ONLY
3
V
CONTAINS DEHP
Hospira
IM - 3797
HOSPIRA, INC., LAKE FOREST, IL 60045 USA

PRINCIPAL DISPLAY PANEL - 250 mL Bag Label
250 mL
NDC 0409-7715-12
20% MANNITOL I.V.
MANNITOL INJECTION, USP
50 g/250 mL (200 mg/mL)
EACH 100 ML CONTAINS MANNITOL
20 GRAMS. MAY CONTAIN SODIUM
BICARBONATE FOR pH ADJUSTMENT.
1098 mOsmol/LITER (CALC.)
pH 6.3 (4.5 TO 7.0)
STERILE, NONPYROGENIC
ADDITIVES MAY BE INCOMPATIBLE.
CONSULT WITH PHARMACIST, IF
AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE,
MIX THOROUGHLY AND DO NOT STORE.
SINGLE-DOSE CONTAINER. FOR
INTRAVENOUS USE. USUAL DOSAGE:
SEE INSERT. WARNING: SEE INSERT
REGARDING CONTRAINDICATION IN
SEVERE RENAL IMPAIRMENT. WARNING:
IF CRYSTALS FORM, WARM WITH
OVERWRAP INTACT AND AGITATE TO
DISSOLVE. ADMINISTER INTRAVENOUSLY
AT 30° TO 37°C (86° TO 98.6°F). INSPECT
CAREFULLY. DISCARD UNUSED PORTION.
USE ONLY IF SOLUTION IS CLEAR AND
CONTAINER IS UNDAMAGED. MUST NOT
BE USED IN SERIES CONNECTIONS.
3
V
Rx ONLY
IM-3799
CONTAINS DEHP
LAKE FOREST, IL 60045 USA
Hospira

PRINCIPAL DISPLAY PANEL - 100 g/500 mL Bag Label
500 mL
NDC 0409-7715-13
20% MANNITOL I.V.
MANNITOL INJECTION, USP
100 g/500 mL (200 mg/mL)
EACH 100 mL CONTAINS MANNITOL 20 GRAMS. MAY CONTAIN
SODIUM BICARBONATE FOR pH ADJUSTMENT.
1098 mOsmol/LITER (CALC.)
STERILE, NONPYROGENIC
pH 6.3 (4.5 TO 7.0)
ADDITIVES MAY BE INCOMPATIBLE. CONSULT WITH
PHARMACIST, IF AVAILABLE. WHEN INTRODUCING
ADDITIVES, USE ASEPTIC TECHNIQUE, MIX THOROUGHLY
AND DO NOT STORE.
SINGLE-DOSE CONTAINER. FOR INTRAVENOUS USE. USUAL
DOSAGE: SEE INSERT. WARNING: SEE INSERT REGARDING
CONTRAINDICATION IN SEVERE RENAL IMPAIRMENT.
WARNING: IF CRYSTALS FORM, WARM WITH OVERWRAP
INTACT AND AGITATE TO DISSOLVE. ADMINISTER
INTRAVENOUSLY AT 30° TO 37°C (86° TO 98.6°F). INSPECT
CAREFULLY. DISCARD UNUSED PORTION. USE ONLY IF
SOLUTION IS CLEAR AND CONTAINER IS UNDAMAGED. MUST
NOT BE USED IN SERIES CONNECTIONS.
3
V
Rx ONLY
IM-3798
CONTAINS DEHP
Hospira
HOSPIRA, INC., LAKE FOREST, IL 60045 USA

| MANNITOL
mannitol injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| MANNITOL
mannitol injection, solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |