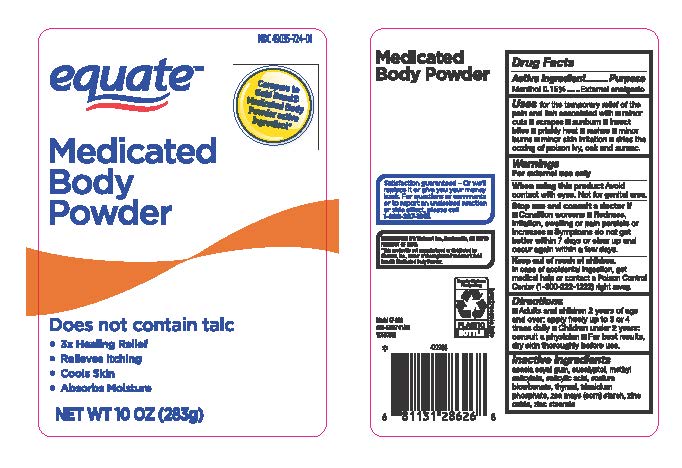
MEDICATED BODY POWDER- menthol powder
Walmart Stores Inc
----------
Equated Medicated Body Powder - 202
Uses
for the temporary relief of the pain and itch associated with
- minor cuts
- scrapes
- sunburn
- insect bites
- prickly heat
- rashes
- minor burns
- minor skin irritations
- dries the oozing of poison ivy, oak and sumac.
Stop use and ask a doctor if
- condition worsens
- Redness, irritation, swelling or pain persist or increases
- symptoms do not get better within 7 days or clear up and occur again within a few days
Keep out of reach of children
Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- adults and children 2 years of age and over, apply freely up to 3 or 4 times daily
- Children under 2 years: consult a physician
- for best results, dry skin thoroughly before use
| MEDICATED BODY
POWDER
menthol powder |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Walmart Stores Inc (051957769) |
Revised: 4/2024
Document Id: 1624d107-d980-d7c0-e063-6294a90a7831
Set id: afd337b7-d76e-f17a-e053-2a95a90abac7
Version: 2
Effective Time: 20240415
Walmart Stores Inc