SEBEX ANTIDANDRUFF, ANTI-SEBORRHEIC DERMATITIS, ANTI-PSORIASIS- salicylic acid, sulfur shampoo
Rugby Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
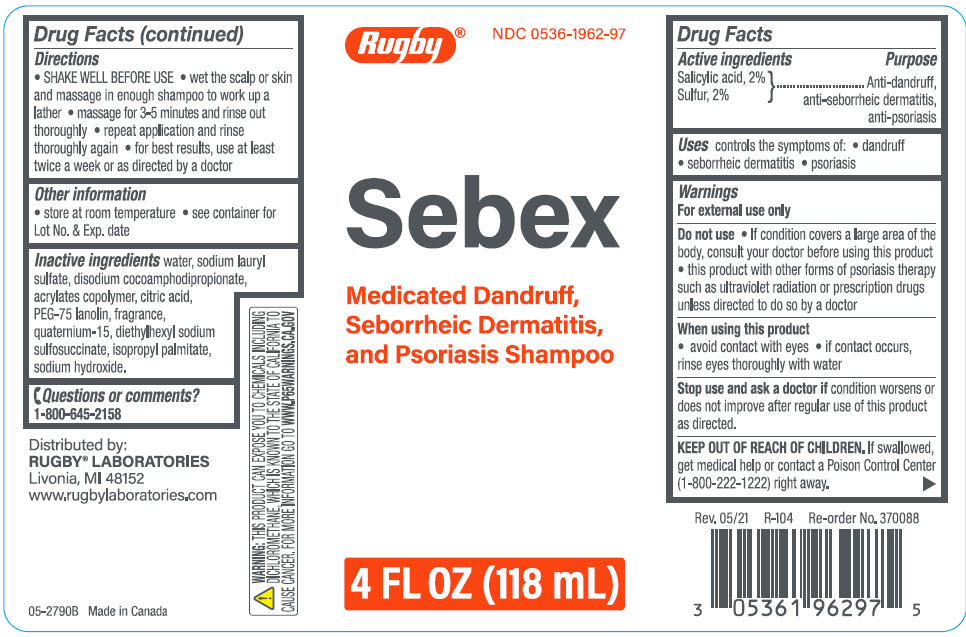
Rugby Sebex Medicated Dandruff, Seborrheic Dermatitis, and Psoriasis Shampoo
Warnings
For external use only
Do not use
- If condition covers a large area of the body, consult your doctor before using this product
- this product with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor
Directions
- SHAKE WELL BEFORE USE
- wet the scalp or skin and massage in enough shampoo to work up a lather
- massage for 3-5 minutes and rinse out thoroughly
- repeat application and rinse thoroughly again
- for best results, use at least twice a week or as directed by a doctor
| SEBEX
ANTIDANDRUFF, ANTI-SEBORRHEIC DERMATITIS, ANTI-PSORIASIS
salicylic acid, sulfur shampoo |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
| Registrant - Garcoa, Inc. (036464697) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sigan Industries, Inc. | 255106239 | MANUFACTURE(0536-1962) | |
Revised: 1/2022
Document Id: ccd4e61e-a839-4a49-a6d5-d9c4b9afd118
Set id: aec81d61-1d28-490f-9654-f66bd4a30ffa
Version: 5
Effective Time: 20220113
Rugby Laboratories