MONTELUKAST SODIUM- montelukast sodium granule
Teva Pharmaceuticals USA, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MONTELUKAST SODIUM ORAL GRANULES safely and effectively. See full prescribing information for MONTELUKAST SODIUM ORAL GRANULES.
MONTELUKAST SODIUM oral granules, for oral use Initial U.S. Approval: 1998 WARNING: SERIOUS NEUROPSYCHIATRIC EVENTSSee full prescribing information for complete boxed warning.
RECENT MAJOR CHANGESINDICATIONS AND USAGEMontelukast sodium is a leukotriene receptor antagonist indicated for:
Limitations of Use:
DOSAGE AND ADMINISTRATIONAdministration (by indications):
Dosage (by age):
Patients with both asthma and allergic rhinitis should take only one dose daily in the evening (2.4). For oral granules: Must administer within 15 minutes after opening the packet (with or without mixing with food) (2.5). DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSHypersensitivity to any component of montelukast sodium oral granules (4). WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥5% and greater than placebo listed in descending order of frequency): upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 5/2021 |
FULL PRESCRIBING INFORMATION
WARNING: SERIOUS NEUROPSYCHIATRIC EVENTS
Serious neuropsychiatric (NP) events have been reported with the use of montelukast sodium. The types of events reported were highly variable, and included, but were not limited to, agitation, aggression, depression, sleep disturbances, suicidal thoughts and behavior (including suicide). The mechanisms underlying NP events associated with montelukast sodium use are currently not well understood [see Warnings and Precautions (5.1)].
Because of the risk of NP events, the benefits of montelukast sodium may not outweigh the risks in some patients, particularly when the symptoms of disease may be mild and adequately treated with alternative therapies. Reserve use of montelukast sodium for patients with allergic rhinitis who have an inadequate response or intolerance to alternative therapies [see Indications and Usage (1.3)]. In patients with asthma, consider the benefits and risks before prescribing montelukast sodium.
Discuss the benefits and risks of montelukast sodium with patients and caregivers when prescribing montelukast sodium. Advise patients and/or caregivers to be alert for changes in behavior or new NP symptoms when taking montelukast sodium. If changes in behavior are observed, or if new NP symptoms or suicidal thoughts and/or behavior occur, advise patients to discontinue montelukast sodium and contact a healthcare provider immediately [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
1.1 Asthma
Montelukast sodium oral granules are indicated for the prophylaxis and chronic treatment of asthma in adults and pediatric patients 12 months of age and older.
1.3 Allergic Rhinitis
Montelukast sodium oral granules are indicated for the relief of symptoms of seasonal allergic rhinitis in patients 2 years of age and older and perennial allergic rhinitis in patients 6 months of age and older. Because the benefits of montelukast sodium oral granules may not outweigh the risk of neuropsychiatric symptoms in patients with allergic rhinitis [see Warnings and Precautions (5.1)], reserve use for patients who have an inadequate response or intolerance to alternative therapies.
2 DOSAGE AND ADMINISTRATION
2.1 Asthma
For asthma, administer montelukast sodium oral granules orally once daily in the evening, with or without food. There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing.
The following doses are recommended:
|
Age |
Dose |
|
Pediatric patients 2 to 5 years of age |
one packet of 4 mg oral granules |
|
Pediatric patients 12 to 23 months of age* |
one packet of 4 mg oral granules |
|
* Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established. |
|
Patients who miss a dose should take the next dose at their regular time and should not take 2 doses at the same time.
2.3 Allergic Rhinitis
For allergic rhinitis, administer montelukast sodium oral granules orally once daily without regard to time of food ingestion. Time of administration in patients with allergic rhinitis can be individualized to suit patient needs.
The following doses for the treatment of symptoms of seasonal allergic rhinitis are recommended:
|
Age |
Dose |
|
Pediatric patients 2 to 5 years of age* |
one packet of 4 mg oral granules |
|
* Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established. |
|
The following doses for the treatment of symptoms of perennial allergic rhinitis are recommended:
|
Age |
Dose |
|
Pediatric patients 2 to 5 years of age |
one packet of 4 mg oral granules |
|
Pediatric patients 6 to 23 months of age* |
one packet of 4 mg oral granules |
|
* Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established. |
|
2.4 Asthma and Allergic Rhinitis
For patients with both asthma and allergic rhinitis, administer only one montelukast sodium oral granule dose orally once daily in the evening.
Patients who miss a dose should take the next dose at their regular time and should not take 2 doses at the same time.2.5 Instructions for Administration of Oral Granules
Montelukast sodium oral granules, 4 mg (montelukast) can be administered either directly in the mouth, dissolved in 1 teaspoonful (5 mL) of cold or room temperature baby formula or breast milk, or mixed with a spoonful of cold or room temperature soft foods; based on stability studies, only applesauce, carrots, rice, or ice cream should be used. The packet should not be opened until ready to use. After opening the packet, the full dose (with or without mixing with baby formula, breast milk, or food) must be administered within 15 minutes. If mixed with baby formula, breast milk, or food, montelukast sodium oral granules must not be stored for future use. Discard any unused portion. Montelukast sodium oral granules are not intended to be dissolved in any liquid other than baby formula or breast milk for administration. However, liquids may be taken subsequent to administration. Montelukast sodium oral granules can be administered without regard to the time of meals.
3 DOSAGE FORMS AND STRENGTHS
- Montelukast sodium oral granules USP, 4 mg (montelukast) are white to slightly yellow granules with 500 mg net weight, packed in a child-resistant foil packet.
4 CONTRAINDICATIONS
Montelukast sodium oral granules are contraindicated in patients with hypersensitivity to any of its components.
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Events
Serious neuropsychiatric (NP) events have been reported with use of montelukast sodium. These postmarketing reports have been highly variable and included, but were not limited to, agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, dysphemia (stuttering), hallucinations, insomnia, irritability, memory impairment, obsessive-compulsive symptoms, restlessness, somnambulism, suicidal thoughts and behavior (including suicide), tic, and tremor. NP events have been reported in adult, adolescent, and pediatric patients with and without a previous history of psychiatric disorder. NP events have been reported mostly during montelukast sodium treatment, but some were reported after montelukast sodium discontinuation. Animal studies showed that montelukast distributes into the brain in rats [see Clinical Pharmacology (12.3)]; however, the mechanisms underlying montelukast sodium-associated NP events are currently not well understood. Based upon the available data, it is difficult to identify risk factors for or quantify the risk of NP events with montelukast sodium use.
Because of the risk of NP events, the benefits of montelukast sodium may not outweigh the risks in some patients, particularly when the symptoms of disease may be mild and adequately treated with alternative therapies. Reserve use of montelukast sodium for patients with allergic rhinitis who have an inadequate response or intolerance to alternative therapies [see Indications and Usage (1.3)]. In patients with asthma, consider the benefits and risks before prescribing montelukast sodium.
Discuss the benefits and risks of montelukast sodium use with patients and caregivers when prescribing montelukast sodium. Advise patients and/or caregivers to be alert for changes in behavior or for new NP symptoms when taking montelukast sodium. If changes in behavior are observed, or if new NP symptoms or suicidal thoughts and/or behavior occur, advise patients to discontinue montelukast sodium and contact a healthcare provider immediately. In many cases, symptoms resolved after stopping montelukast sodium therapy; however, in some cases symptoms persisted after discontinuation of montelukast sodium. Therefore, continue to monitor and provide supportive care until symptoms resolve. Re-evaluate the benefits and risks of restarting treatment with montelukast sodium if such events occur.
5.2 Acute Asthma
Montelukast sodium is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available. Therapy with montelukast sodium can be continued during acute exacerbations of asthma. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled β-agonist.
5.3 Concomitant Corticosteroid Use
While the dose of inhaled corticosteroid may be reduced gradually under medical supervision, montelukast sodium should not be abruptly substituted for inhaled or oral corticosteroids.
5.4 Aspirin Sensitivity
Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast sodium. Although montelukast sodium is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate bronchoconstrictor response to aspirin and other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients [see Clinical Studies (14.1)].
5.5 Eosinophilic Conditions
Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between montelukast sodium and these underlying conditions has not been established [see Adverse Reactions (6.2)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Neuropsychiatric Events [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the following description of clinical trials experience, adverse reactions are listed regardless of causality assessment.
The most common adverse reactions (incidence ≥5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.
Adults and Adolescents 15 Years of Age and Older with Asthma
Montelukast sodium has been evaluated for safety in approximately 2950 adult and adolescent patients 15 years of age and older in clinical trials. In placebo-controlled clinical trials, the following adverse reactions reported with montelukast sodium occurred in greater than or equal to 1% of patients and at an incidence greater than that in patients treated with placebo:
|
Montelukast 10 mg/day (%) (n=1955) |
Placebo (%) (n=1180) |
|
|
Body As A Whole | ||
|
Pain, abdominal |
2.9 |
2.5 |
|
Asthenia/fatigue |
1.8 |
1.2 |
|
Fever |
1.5 |
0.9 |
|
Trauma |
1.0 |
0.8 |
|
Digestive System Disorders | ||
|
Dyspepsia |
2.1 |
1.1 |
|
Pain, dental |
1.7 |
1.0 |
|
Gastroenteritis, infectious |
1.5 |
0.5 |
|
Nervous System/Psychiatric | ||
|
Headache |
18.4 |
18.1 |
|
Dizziness |
1.9 |
1.4 |
|
Respiratory System Disorders | ||
|
Influenza |
4.2 |
3.9 |
|
Cough |
2.7 |
2.4 |
|
Congestion, nasal |
1.6 |
1.3 |
|
Skin/Skin Appendages Disorder | ||
|
Rash |
1.6 |
1.2 |
|
Laboratory Adverse Reactions* | ||
|
ALT increased |
2.1 |
2.0 |
|
AST increased |
1.6 |
1.2 |
|
Pyuria |
1.0 |
0.9 |
|
* Number of patients tested (montelukast sodium and placebo, respectively): ALT and AST, 1935, 1170; pyuria, 1924, 1159. |
||
The frequency of less common adverse reactions was comparable between montelukast sodium and placebo.
Cumulatively, 569 patients were treated with montelukast sodium for at least 6 months, 480 for one year, and 49 for two years in clinical trials. With prolonged treatment, the adverse reaction profile did not significantly change.
Pediatric Patients 6 to 14 Years of Age with Asthma
Montelukast sodium has been evaluated for safety in 476 pediatric patients 6 to 14 years of age. Cumulatively, 289 pediatric patients were treated with montelukast sodium for at least 6 months, and 241 for one year or longer in clinical trials. The safety profile of montelukast sodium in the 8-week, double-blind, pediatric efficacy trial was generally similar to the adult safety profile. In pediatric patients 6 to 14 years of age receiving montelukast sodium, the following reactions occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: pharyngitis, influenza, fever, sinusitis, nausea, diarrhea, dyspepsia, otitis, viral infection, and laryngitis. The frequency of less common adverse reactions was comparable between montelukast sodium and placebo. With prolonged treatment, the adverse reaction profile did not significantly change.
In studies evaluating growth rate, the safety profile in these pediatric patients was consistent with the safety profile previously described for montelukast sodium. In a 56-week, double-blind study evaluating growth rate in pediatric patients 6 to 8 years of age receiving montelukast sodium, the following reactions not previously observed with the use of montelukast sodium in this age group occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: headache, rhinitis (infective), varicella, gastroenteritis, atopic dermatitis, acute bronchitis, tooth infection, skin infection, and myopia.
Pediatric Patients 2 to 5 Years of Age with Asthma
Montelukast sodium has been evaluated for safety in 573 pediatric patients 2 to 5 years of age in single- and multiple-dose studies. Cumulatively, 426 pediatric patients 2 to 5 years of age were treated with montelukast sodium for at least 3 months, 230 for 6 months or longer, and 63 patients for one year or longer in clinical trials. In pediatric patients 2 to 5 years of age receiving montelukast sodium, the following reactions occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: fever, cough, abdominal pain, diarrhea, headache, rhinorrhea, sinusitis, otitis, influenza, rash, ear pain, gastroenteritis, eczema, urticaria, varicella, pneumonia, dermatitis, and conjunctivitis.
Pediatric Patients 6 to 23 Months of Age with Asthma
Safety and effectiveness in pediatric patients younger than 12 months of age with asthma have not been established.
Montelukast sodium has been evaluated for safety in 175 pediatric patients 6 to 23 months of age. The safety profile of montelukast sodium in a 6-week, double-blind, placebo-controlled clinical study was generally similar to the safety profile in adults and pediatric patients 2 to 14 years of age. In pediatric patients 6 to 23 months of age receiving montelukast sodium, the following reactions occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: upper respiratory infection, wheezing; otitis media; pharyngitis, tonsillitis, cough; and rhinitis. The frequency of less common adverse reactions was comparable between montelukast sodium and placebo.
Adults and Adolescents 15 Years of Age and Older with Seasonal Allergic Rhinitis
Montelukast sodium has been evaluated for safety in 2199 adult and adolescent patients 15 years of age and older in clinical trials. Montelukast sodium administered once daily in the morning or in the evening had a safety profile similar to that of placebo. In placebo-controlled clinical trials, the following reaction was reported with montelukast sodium with a frequency ≥1% and at an incidence greater than placebo: upper respiratory infection, 1.9% of patients receiving montelukast sodium vs. 1.5% of patients receiving placebo. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with that observed in 2-week studies. The incidence of somnolence was similar to that of placebo in all studies.
Pediatric Patients 2 to 14 Years of Age with Seasonal Allergic Rhinitis
Montelukast sodium has been evaluated in 280 pediatric patients 2 to 14 years of age in a 2-week, multicenter, double-blind, placebo-controlled, parallel-group safety study. Montelukast sodium administered once daily in the evening had a safety profile similar to that of placebo. In this study, the following reactions occurred with a frequency ≥2% and at an incidence greater than placebo: headache, otitis media, pharyngitis, and upper respiratory infection.
Adults and Adolescents 15 Years of Age and Older with Perennial Allergic Rhinitis
Montelukast sodium has been evaluated for safety in 3357 adult and adolescent patients 15 years of age and older with perennial allergic rhinitis of whom 1632 received montelukast sodium in two, 6-week, clinical studies. Montelukast sodium administered once daily had a safety profile consistent with that observed in patients with seasonal allergic rhinitis and similar to that of placebo. In these two studies, the following reactions were reported with montelukast sodium with a frequency ≥1% and at an incidence greater than placebo: sinusitis, upper respiratory infection, sinus headache, cough, epistaxis, and increased ALT. The incidence of somnolence was similar to that of placebo.
Pediatric Patients 6 Months to 14 Years of Age with Perennial Allergic Rhinitis
The safety in patients 2 to 14 years of age with perennial allergic rhinitis is supported by the safety in patients 2 to 14 years of age with seasonal allergic rhinitis. The safety in patients 6 to 23 months of age is supported by data from pharmacokinetic and safety and efficacy studies in asthma in this pediatric population and from adult pharmacokinetic studies.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of montelukast sodium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders
increased bleeding tendency, thrombocytopenia
Immune system disorders
hypersensitivity reactions including anaphylaxis, hepatic eosinophilic infiltration
Psychiatric disorders
including, but not limited to, agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, dysphemia (stuttering), hallucinations, insomnia, irritability, memory impairment, obsessive-compulsive symptoms, restlessness, somnambulism, suicidal thinking and behavior (including suicide), tic, and tremor [see Boxed Warning, Warnings and Precautions (5.1)]
Nervous system disorders
drowsiness, paraesthesia/hypoesthesia, seizures
Cardiac disorders
palpitations
Respiratory, thoracic and mediastinal disorders
epistaxis, pulmonary eosinophilia
Gastrointestinal disorders
diarrhea, dyspepsia, nausea, pancreatitis, vomiting
Hepatobiliary disorders
Cases of cholestatic hepatitis, hepatocellular liver-injury, and mixed-pattern liver injury have been reported in patients treated with montelukast sodium. Most of these occurred in combination with other confounding factors, such as use of other medications, or when montelukast sodium was administered to patients who had underlying potential for liver disease such as alcohol use or other forms of hepatitis.
Skin and subcutaneous tissue disorders
angioedema, bruising, erythema multiforme, erythema nodosum, pruritus, Stevens-Johnson syndrome/toxic epidermal necrolysis, urticaria
Musculoskeletal and connective tissue disorders
arthralgia, myalgia including muscle cramps
Renal and urinary disorders
enuresis in children
General disorders and administration site conditions
edema
Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These reactions have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients [see Warnings and Precautions (5.5)].
7 DRUG INTERACTIONS
No dose adjustment is needed when montelukast sodium is coadministered with theophylline, prednisone, prednisolone, oral contraceptives, fexofenadine, digoxin, warfarin, gemfibrozil, itraconazole, thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, decongestants, and Cytochrome P450 (CYP) enzyme inducers [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published prospective and retrospective cohort studies over decades with montelukast use in pregnant women have not established a drug-associated risk of major birth defects [see Data]. In animal reproduction studies, no adverse developmental effects were observed with oral administration of montelukast to pregnant rats and rabbits during organogenesis at doses approximately 100 and 110 times, respectively, the maximum recommended human daily oral dose (MRHDOD) based on AUCs [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly or moderately controlled asthma in pregnancy increases the maternal risk of perinatal adverse outcomes such as preeclampsia and infant prematurity, low birth weight, and small for gestational age.
Data
Human Data
Published data from prospective and retrospective cohort studies have not identified an association with montelukast use during pregnancy and major birth defects. Available studies have methodologic limitations, including small sample size, in some cases retrospective data collection, and inconsistent comparator groups.
Animal Data
In embryo-fetal development studies, montelukast administered to pregnant rats and rabbits during organogenesis (gestation days 6 to 17 in rats and 6 to 18 in rabbits) did not cause any adverse developmental effects at maternal oral doses up to 400 and 300 mg/kg/day in rats and rabbits, respectively (approximately 100 and 110 times the AUC in humans at the MRHDOD, respectively).
8.2 Lactation
Risk Summary
A published clinical lactation study reports the presence of montelukast in human milk. Data available on the effects of the drug on infants, either directly [see Use in Specific Populations (8.4)] or through breast milk, do not suggest a significant risk of adverse reactions from exposure to montelukast. The effects of the drug on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for montelukast and any potential adverse reactions on the breastfed infant from montelukast or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of montelukast sodium for asthma have been established in pediatric patients 6 to 14 years of age. Use of montelukast sodium for this indication is supported by evidence from well-controlled studies. Safety and efficacy data in this age group are similar to those seen in adults [see Adverse Reactions (6.1), Clinical Pharmacology, Specific Populations (12.3), and Clinical Studies (14.1)].
The effectiveness of montelukast sodium for the treatment of seasonal allergic rhinitis in pediatric patients 2 to 14 years of age and for the treatment of perennial allergic rhinitis in pediatric patients 6 months to 14 years of age have been established and is supported by extrapolation from the demonstrated effectiveness in patients 15 years of age and older with allergic rhinitis as well as the assumption that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations.
The safety of montelukast sodium oral granules, 4 mg in pediatric patients 12 to 23 months of age with asthma has been demonstrated in an analysis of 172 pediatric patients, 124 of whom were treated with montelukast sodium, in a 6-week, double-blind, placebo-controlled study [see Adverse Reactions (6.1)]. Effectiveness of montelukast sodium in this age group is extrapolated from the demonstrated effectiveness in patients 6 years of age and older with asthma based on similar mean systemic exposure (AUC), and that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations, supported by efficacy data from a safety trial in which efficacy was an exploratory assessment.
The safety of montelukast sodium oral granules, 4 mg in pediatric patients as young as 6 months of age with perennial allergic rhinitis is supported by extrapolation from safety data obtained from studies conducted in pediatric patients 6 months to 23 months of age with asthma and from pharmacokinetic data comparing systemic exposures in patients 6 months to 23 months of age to systemic exposures in adults.
The safety and effectiveness in pediatric patients below the age of 12 months with asthma and 6 months with perennial allergic rhinitis have not been established.
Growth Rate in Pediatric Patients
A 56-week, multi-center, double-blind, randomized, active- and placebo-controlled parallel group study was conducted to assess the effect of montelukast sodium on growth rate in 360 patients with mild asthma, aged 6 to 8 years. Treatment groups included montelukast 5 mg once daily, placebo, and beclomethasone dipropionate administered as 168 mcg twice daily with a spacer device. For each subject, a growth rate was defined as the slope of a linear regression line fit to the height measurements over 56 weeks. The primary comparison was the difference in growth rates between montelukast sodium and placebo groups. Growth rates, expressed as least-squares (LS) mean (95% CI) in cm/year, for the montelukast sodium, placebo, and beclomethasone treatment groups were 5.67 (5.46, 5.88), 5.64 (5.42, 5.86), and 4.86 (4.64, 5.08), respectively. The differences in growth rates, expressed as least-squares (LS) mean (95% CI) in cm/year, for montelukast sodium minus placebo, beclomethasone minus placebo, and montelukast sodium minus beclomethasone treatment groups were 0.03 (-0.26, 0.31), -0.78 (-1.06, -0.49); and 0.81 (0.53, 1.09), respectively. Growth rate (expressed as mean change in height over time) for each treatment group is shown in FIGURE 1.
Figure 1: Change in Height (cm) from Randomization Visit by Scheduled Week (Treatment Group Mean ± Standard Error* of the Mean)
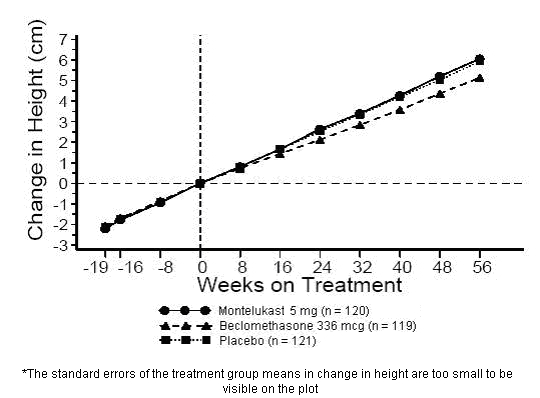
8.5 Geriatric Use
Of the total number of subjects in clinical studies of montelukast, 3.5% were 65 years of age and over, and 0.4% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetic profile and the oral bioavailability of a single 10 mg oral dose of montelukast are similar in elderly and younger adults. The plasma half-life of montelukast is slightly longer in the elderly. No dosage adjustment in the elderly is required.
8.6 Hepatic Impairment
No dosage adjustment is recommended in patients with mild-to-moderate hepatic insufficiency [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment is recommended in patients with renal insufficiency [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
No specific information is available on the treatment of overdosage with montelukast sodium. In the event of overdose, it is reasonable to employ the usual supportive measures; e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive therapy, if required. It is not known whether montelukast is removed by peritoneal dialysis or hemodialysis.
11 DESCRIPTION
Montelukast sodium, USP the active ingredient in montelukast sodium oral granules, USP is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl leukotriene CysLT1 receptor.
Montelukast sodium, USP is described chemically as sodium 1-[[[(R)-m-[(E)-2-(7-chloro-2-quinolyl)vinyl]-α-[o-(1-hydroxy-1-methylethyl)phenethyl]benzyl]thio]-methyl]cyclopropaneaceacetate.
The structural formula is:
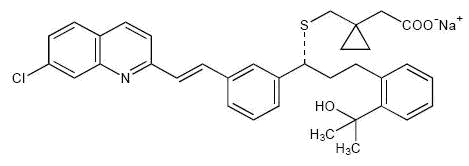
C35H35ClNNaO3S M.W. 608.17
Montelukast sodium, USP is a hygroscopic, optically active, white to off-white to light yellow powder. Montelukast sodium, USP is freely soluble to very soluble in alcohol; freely soluble in water and in methylene chloride.
Each packet of montelukast sodium oral granules USP, 4 mg contains 4.2 mg montelukast sodium, USP, which is equivalent to 4 mg of montelukast. The oral granule formulation contains the following inactive ingredients: hydroxypropyl cellulose, magnesium stearate, mannitol, and sodium lauryl sulfate.
This product meets USP Dissolution Test 3.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The cysteinyl leukotrienes (LTC4, LTD4, LTE4) are products of arachidonic acid metabolism and are released from various cells, including mast cells and eosinophils. These eicosanoids bind to cysteinyl leukotriene (CysLT) receptors. The CysLT type-1 (CysLT1) receptor is found in the human airway (including airway smooth muscle cells and airway macrophages) and on other pro-inflammatory cells (including eosinophils and certain myeloid stem cells). CysLTs have been correlated with the pathophysiology of asthma and allergic rhinitis. In asthma, leukotriene-mediated effects include airway edema, smooth muscle contraction, and altered cellular activity associated with the inflammatory process. In allergic rhinitis, CysLTs are released from the nasal mucosa after allergen exposure during both early- and late-phase reactions and are associated with symptoms of allergic rhinitis.
Montelukast is an orally active compound that binds with high affinity and selectivity to the CysLT1 receptor (in preference to other pharmacologically important airway receptors, such as the prostanoid, cholinergic, or β-adrenergic receptor). Montelukast inhibits physiologic actions of LTD4 at the CysLT1 receptor without any agonist activity.
12.2 Pharmacodynamics
Montelukast causes inhibition of airway cysteinyl leukotriene receptors as demonstrated by the ability to inhibit bronchoconstriction due to inhaled LTD4 in asthmatics. Doses as low as 5 mg cause substantial blockage of LTD4-induced bronchoconstriction. In a placebo-controlled, crossover study (n=12), montelukast sodium inhibited early- and late-phase bronchoconstriction due to antigen challenge by 75% and 57%, respectively.
The effect of montelukast sodium on eosinophils in the peripheral blood was examined in clinical trials. In patients with asthma aged 2 years and older who received montelukast sodium, a decrease in mean peripheral blood eosinophil counts ranging from 9% to 15% was noted, compared with placebo, over the double-blind treatment periods. In patients with seasonal allergic rhinitis aged 15 years and older who received montelukast sodium, a mean increase of 0.2% in peripheral blood eosinophil counts was noted, compared with a mean increase of 12.5% in placebo-treated patients, over the double-blind treatment periods; this reflects a mean difference of 12.3% in favor of montelukast sodium. The relationship between these observations and the clinical benefits of montelukast noted in the clinical trials is not known [see Clinical Studies (14)].
12.3 Pharmacokinetics
Absorption
Montelukast is rapidly absorbed following oral administration. After administration of the 10 mg film-coated tablet to fasted adults, the mean peak montelukast plasma concentration (Cmax) is achieved in 3 to 4 hours (Tmax). The mean oral bioavailability is 64%. The oral bioavailability and Cmax are not influenced by a standard meal in the morning.
For the 5 mg chewable tablet, the mean Cmax is achieved in 2 to 2.5 hours after administration to adults in the fasted state. The mean oral bioavailability is 73% in the fasted state versus 63% when administered with a standard meal in the morning.
For the 4 mg chewable tablet, the mean Cmax is achieved 2 hours after administration in pediatric patients 2 to 5 years of age in the fasted state.
The 4 mg oral granule formulation is bioequivalent to the 4 mg chewable tablet when administered to adults in the fasted state. The coadministration of the oral granule formulation with applesauce did not have a clinically significant effect on the pharmacokinetics of montelukast. A high fat meal in the morning did not affect the AUC of montelukast oral granules; however, the meal decreased Cmax by 35% and prolonged Tmax from 2.3 ± 1.0 hours to 6.4 ± 2.9 hours.
The safety and effectiveness of montelukast sodium in patients with asthma were demonstrated in clinical trials in which the 10 mg film-coated tablet and 5 mg chewable tablet formulations were administered in the evening without regard to the time of food ingestion. The safety of montelukast in patients with asthma was also demonstrated in clinical trials in which the 4 mg chewable tablet and 4 mg oral granule formulations were administered in the evening without regard to the time of food ingestion. The safety and effectiveness of montelukast in patients with seasonal allergic rhinitis were demonstrated in clinical trials in which the 10 mg film-coated tablet was administered in the morning or evening without regard to the time of food ingestion.
The comparative pharmacokinetics of montelukast when administered as two 5 mg chewable tablets versus one 10 mg film-coated tablet have not been evaluated.
Distribution
Montelukast is more than 99% bound to plasma proteins. The steady state volume of distribution of montelukast averages 8 to 11 liters. Orally administered montelukast distributes into the brain in rats.
Elimination
The plasma clearance of montelukast averages 45 mL/min in healthy adults. Following an oral dose of radiolabeled montelukast, 86% of the radioactivity was recovered in 5-day fecal collections and <0.2% was recovered in urine. Coupled with estimates of montelukast oral bioavailability, this indicates that montelukast and its metabolites are excreted almost exclusively via the bile.
In several studies, the mean plasma half-life of montelukast ranged from 2.7 to 5.5 hours in healthy young adults. The pharmacokinetics of montelukast are nearly linear for oral doses up to 50 mg. During once-daily dosing with 10 mg montelukast, there is little accumulation of the parent drug in plasma (14%).
Metabolism
Montelukast is extensively metabolized. In studies with therapeutic doses, plasma concentrations of metabolites of montelukast are undetectable at steady state in adults and pediatric patients.
In vitro studies using human liver microsomes indicate that CYP3A4, 2C8, and 2C9 are involved in the metabolism of montelukast. At clinically relevant concentrations, 2C8 appears to play a major role in the metabolism of montelukast.
Specific Populations
Patients with Hepatic Impairment
Patients with mild-to-moderate hepatic insufficiency and clinical evidence of cirrhosis had evidence of decreased metabolism of montelukast resulting in 41% (90% CI=7%, 85%) higher mean montelukast AUC following a single 10 mg dose. The elimination of montelukast was slightly prolonged compared with that in healthy subjects (mean half-life, 7.4 hours). No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency. The pharmacokinetics of montelukast sodium in patients with more severe hepatic impairment or with hepatitis have not been evaluated.
Patients with Renal Impairment
Since montelukast and its metabolites are not excreted in the urine, the pharmacokinetics of montelukast were not evaluated in patients with renal insufficiency. No dosage adjustment is recommended in these patients.
Male and Female Patients
The pharmacokinetics of montelukast are similar in males and females.
Racial Groups
Pharmacokinetic differences due to race have not been studied.
Adolescents and Pediatric Patients
Pharmacokinetic studies evaluated the systemic exposure of the 4 mg oral granule formulation in pediatric patients 6 to 23 months of age.
In children 6 to 11 months of age, the systemic exposure to montelukast and the variability of plasma montelukast concentrations were higher than those observed in adults. Based on population analyses, the mean AUC (4296 ng•hr/mL [range 1200 to 7153]) was 60% higher and the mean Cmax (667 ng/mL [range 201 to 1058]) was 89% higher than those observed in adults (mean AUC 2689 ng•hr/mL [range 1521 to 4595]) and mean Cmax (353 ng/mL [range 180 to 548]). The systemic exposure in children 12 to 23 months of age was less variable, but was still higher than that observed in adults. The mean AUC (3574 ng•hr/mL [range 2229 to 5408]) was 33% higher and the mean Cmax (562 ng/mL [range 296 to 814]) was 60% higher than those observed in adults. Safety and tolerability of montelukast in a single-dose pharmacokinetic study in 26 children 6 to 23 months of age were similar to that of patients two years and above [see Adverse Reactions (6.1)]. The 4 mg oral granule formulation should be used for pediatric patients 12 to 23 months of age for the treatment of asthma, or for pediatric patients 6 to 23 months of age for the treatment of perennial allergic rhinitis. Since the 4 mg oral granule formulation is bioequivalent to the 4 mg chewable tablet, it can also be used as an alternative formulation to the 4 mg chewable tablet in pediatric patients 2 to 5 years of age.
Drug Interaction Studies
Theophylline, Prednisone, and Prednisolone
Montelukast sodium has been administered with other therapies routinely used in the prophylaxis and chronic treatment of asthma with no apparent increase in adverse reactions. In drug-interaction studies, the recommended clinical dose of montelukast did not have clinically important effects on the pharmacokinetics of the following drugs: theophylline, prednisone, and prednisolone.
Montelukast at a dose of 10 mg once daily dosed to pharmacokinetic steady state, did not cause clinically significant changes in the kinetics of a single intravenous dose of theophylline [predominantly a cytochrome P450 (CYP) 1A2 substrate]. Montelukast at doses of ≥100 mg daily dosed to pharmacokinetic steady state, did not cause any clinically significant change in plasma profiles of prednisone or prednisolone following administration of either oral prednisone or intravenous prednisolone.
Oral Contraceptives, fexofenadine, Digoxin, and Warfarin
In drug interaction studies, the recommended clinical dose of montelukast did not have clinically important effects on the pharmacokinetics of the following drugs: oral contraceptives (norethindrone 1 mg/ethinyl estradiol 35 mcg), digoxin, and warfarin. Montelukast at doses of ≥100 mg daily dosed to pharmacokinetic steady state did not significantly alter the plasma concentrations of either component of an oral contraceptive containing norethindrone 1 mg/ethinyl estradiol 35 mcg. Montelukast at a dose of 10 mg once daily dosed to pharmacokinetic steady state did not change the plasma concentration profile of fexofenadine, did not change the pharmacokinetic profile or urinary excretion of immunoreactive digoxin; did not change the pharmacokinetic profile of warfarin (primarily a substrate of CYP2C9, 3A4 and 1A2) or influence the effect of a single 30 mg oral dose of warfarin on prothrombin time or the International Normalized Ratio (INR).
Thyroid Hormones, Sedative Hypnotics, Non-Steroidal Anti-Inflammatory Agents, Benzodiazepines, and Decongestants
Although additional specific interaction studies were not performed, montelukast sodium was used concomitantly with a wide range of commonly prescribed drugs in clinical studies without evidence of clinical adverse interactions. These medications included thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, and decongestants.
Cytochrome P450 (CYP) Enzyme Inducers
Phenobarbital, which induces hepatic metabolism, decreased the area under the plasma concentration curve (AUC) of montelukast approximately 40% following a single 10 mg dose of montelukast. No dosage adjustment for montelukast sodium is recommended. It is reasonable to employ appropriate clinical monitoring when potent CYP enzyme inducers, such as phenobarbital or rifampin, are coadministered with montelukast sodium.
Effect of Montelukast on Cytochrome P450 (CYP) Enzymes
Montelukast is a potent inhibitor of CYP2C8 in vitro. However, data from a clinical drug-drug interaction study involving montelukast and rosiglitazone (a probe substrate representative of drugs primarily metabolized by CYP2C8) in 12 healthy individuals demonstrated that the pharmacokinetics of rosiglitazone are not altered when the drugs are coadministered, indicating that montelukast does not inhibit CYP2C8 in vivo. Therefore, montelukast is not anticipated to alter the metabolism of drugs metabolized by this enzyme (e.g., paclitaxel, rosiglitazone, and repaglinide). Based on further in vitro results in human liver microsomes, therapeutic plasma concentrations of montelukast do not inhibit CYP 3A4, 2C9, 1A2, 2A6, 2C19, or 2D6.
Cytochrome P450 (CYP) Enzyme Inhibitors
In vitro studies have shown that montelukast is a substrate of CYP 2C8, 2C9, and 3A4. Coadministration of montelukast with itraconazole, a strong CYP 3A4 inhibitor, resulted in no significant increase in the systemic exposure of montelukast. Data from a clinical drug interaction study involving montelukast and gemfibrozil (an inhibitor of both CYP 2C8 and 2C9) demonstrated that gemfibrozil, at a therapeutic dose, increased the systemic exposure of montelukast by 4.4 fold. Coadministration of itraconazole, gemfibrozil, and montelukast did not further increase the systemic exposure of montelukast. Based on available clinical experience, no dosage adjustment of montelukast is required upon coadministration with gemfibrozil [see Overdosage (10)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of tumorigenicity was seen in carcinogenicity studies of either 2 years in Sprague-Dawley rats or 92 weeks in mice at oral gavage doses up to 200 mg/kg/day or 100 mg/kg/day, respectively. The estimated exposure in rats was approximately 120 and 75 times the AUC for adults and children, respectively, at the maximum recommended daily oral dose. The estimated exposure in mice was approximately 45 and 25 times the AUC for adults and children, respectively, at the maximum recommended daily oral dose.
Montelukast demonstrated no evidence of mutagenic or clastogenic activity in the following assays: the microbial mutagenesis assay, the V-79 mammalian cell mutagenesis assay, the alkaline elution assay in rat hepatocytes, the chromosomal aberration assay in Chinese hamster ovary cells, and in the in vivo mouse bone marrow chromosomal aberration assay.
In fertility studies in female rats, montelukast produced reductions in fertility and fecundity indices at an oral dose of 200 mg/kg (estimated exposure was approximately 70 times the AUC for adults at the maximum recommended daily oral dose). No effects on female fertility or fecundity were observed at an oral dose of 100 mg/kg (estimated exposure was approximately 20 times the AUC for adults at the maximum recommended daily oral dose). Montelukast had no effects on fertility in male rats at oral doses up to 800 mg/kg (estimated exposure was approximately 160 times the AUC for adults at the maximum recommended daily oral dose).
14 CLINICAL STUDIES
14.1 Asthma
Adults and Adolescents 15 Years of Age and Older with Asthma
Clinical trials in adults and adolescents 15 years of age and older demonstrated there is no additional clinical benefit to montelukast doses above 10 mg once daily.
The efficacy of montelukast sodium for the chronic treatment of asthma in adults and adolescents 15 years of age and older was demonstrated in two (U.S. and Multinational) similarly designed, randomized, 12-week, double-blind, placebo-controlled trials in 1576 patients (795 treated with montelukast sodium, 530 treated with placebo, and 251 treated with active control). The median age was 33 years (range 15 to 85); 56.8% were females and 43.2% were males. The ethnic/racial distribution in these studies was 71.6% Caucasian, 17.7% Hispanic, 7.2% other origins and 3.5% Black. Patients had mild or moderate asthma and were non-smokers who required approximately 5 puffs of inhaled β-agonist per day on an “as-needed” basis. The patients had a mean baseline percent of predicted forced expiratory volume in 1 second (FEV1) of 66% (approximate range, 40 to 90%). The co-primary endpoints in these trials were FEV1 and daytime asthma symptoms. In both studies after 12 weeks, a random subset of patients receiving montelukast sodium was switched to placebo for an additional 3 weeks of double-blind treatment to evaluate for possible rebound effects.
The results of the U.S. trial on the primary endpoint, morning FEV1, expressed as mean percent change from baseline averaged over the 12-week treatment period, are shown in FIGURE 2. Compared with placebo, treatment with one montelukast sodium tablet, 10 mg daily in the evening resulted in a statistically significant increase in FEV1 percent change from baseline (13.0% change in the group treated with montelukast sodium vs. 4.2% change in the placebo group, p<0.001); the change from baseline in FEV1 for montelukast sodium was 0.32 liters compared with 0.10 liters for placebo, corresponding to a between-group difference of 0.22 liters (p<0.001, 95% CI 0.17 liters, 0.27 liters). The results of the Multinational trial on FEV1 were similar.
Figure 2: FEV1 Mean Percent Change from Baseline (U.S. Trial: Montelukast Sodium N=406; Placebo N=270) (ANOVA Model)
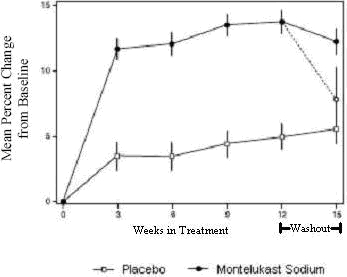
The effect of montelukast sodium on other primary and secondary endpoints, represented by the Multinational study is shown in TABLE 6. Results on these endpoints were similar in the U.S. study.
|
Montelukast Sodium |
Placebo |
|||||
|
Endpoint |
N |
Baseline |
Mean Change from Baseline |
N |
Baseline |
Mean Change from Baseline |
|
Daytime Asthma Symptoms (0 to 6 scale) |
372 |
2.35 |
-0.49* |
245 |
2.40 |
-0.26 |
|
β-agonist (puffs per day) |
371 |
5.35 |
-1.65* |
241 |
5.78 |
-0.42 |
|
AM PEFR (L/min) |
372 |
339.57 |
25.03* |
244 |
335.24 |
1.83 |
|
PM PEFR (L/min) |
372 |
355.23 |
20.13* |
244 |
354.02 |
-0.49 |
|
Nocturnal Awakenings (#/week) |
285 |
5.46 |
-2.03* |
195 |
5.57 |
-0.78 |
|
* p<0.001, compared with placebo |
||||||
Both studies evaluated the effect of montelukast sodium on secondary outcomes, including asthma attack (utilization of health-care resources such as an unscheduled visit to a doctor's office, emergency room, or hospital; or treatment with oral, intravenous, or intramuscular corticosteroid), and use of oral corticosteroids for asthma rescue. In the Multinational study, significantly fewer patients (15.6% of patients) on montelukast sodium experienced asthma attacks compared with patients on placebo (27.3%, p<0.001). In the U.S. study, 7.8% of patients on montelukast sodium and 10.3% of patients on placebo experienced asthma attacks, but the difference between the two treatment groups was not significant (p=0.334). In the Multinational study, significantly fewer patients (14.8% of patients) on montelukast sodium were prescribed oral corticosteroids for asthma rescue compared with patients on placebo (25.7%, p<0.001). In the U.S. study, 6.9% of patients on montelukast sodium and 9.9% of patients on placebo were prescribed oral corticosteroids for asthma rescue, but the difference between the two treatment groups was not significant (p=0.196).
Onset of Action and Maintenance of Effects
In each placebo-controlled trial in adults, the treatment effect of montelukast sodium, measured by daily diary card parameters, including symptom scores, “as-needed” β-agonist use, and PEFR measurements, was achieved after the first dose and was maintained throughout the dosing interval (24 hours). No significant change in treatment effect was observed during continuous once-daily evening administration in non-placebo-controlled extension trials for up to one year. Withdrawal of montelukast sodium in asthmatic patients after 12 weeks of continuous use did not cause rebound worsening of asthma.
Pediatric Patients 6 to 14 Years of Age with Asthma
The efficacy of montelukast sodium in pediatric patients 6 to 14 years of age was demonstrated in one 8-week, double-blind, placebo-controlled trial in 336 patients (201 treated with montelukast sodium and 135 treated with placebo) using an inhaled β-agonist on an “as-needed” basis. The patients had a mean baseline percent predicted FEV1 of 72% (approximate range, 45 to 90%) and a mean daily inhaled β-agonist requirement of 3.4 puffs of albuterol. Approximately 36% of the patients were on inhaled corticosteroids. The median age was 11 years (range 6 to 15); 35.4% were females and 64.6% were males. The ethnic/racial distribution in this study was 80.1% Caucasian, 12.8% Black, 4.5% Hispanic, and 2.7% other origins.
Compared with placebo, treatment with one 5 mg montelukast sodium chewable tablet daily resulted in a significant improvement in mean morning FEV1 percent change from baseline (8.7% in the group treated with montelukast sodium vs. 4.2% change from baseline in the placebo group, p<0.001). There was a significant decrease in the mean percentage change in daily “as-needed” inhaled β-agonist use (11.7% decrease from baseline in the group treated with montelukast sodium vs. 8.2% increase from baseline in the placebo group, p<0.05). This effect represents a mean decrease from baseline of 0.56 and 0.23 puffs per day for the montelukast and placebo groups, respectively. Subgroup analyses indicated that younger pediatric patients aged 6 to 11 had efficacy results comparable to those of the older pediatric patients aged 12 to 14.
Similar to the adult studies, no significant change in the treatment effect was observed during continuous once-daily administration in one open-label extension trial without a concurrent placebo group for up to 6 months.
Pediatric Patients 2 to 5 Years of Age with Asthma
The efficacy of montelukast sodium for the chronic treatment of asthma in pediatric patients 2 to 5 years of age was explored in a 12-week, placebo-controlled safety and tolerability study in 689 patients, 461 of whom were treated with montelukast sodium. The median age was 4 years (range 2 to 6); 41.5% were females and 58.5% were males. The ethnic/racial distribution in this study was 56.5% Caucasian, 20.9% Hispanic, 14.4% other origins, and 8.3% Black.
While the primary objective was to determine the safety and tolerability of montelukast sodium in this age group, the study included exploratory efficacy evaluations, including daytime and overnight asthma symptom scores, β-agonist use, oral corticosteroid rescue, and the physician’s global evaluation. The findings of these exploratory efficacy evaluations, along with pharmacokinetics and extrapolation of efficacy data from older patients, support the overall conclusion that montelukast sodium is efficacious in the maintenance treatment of asthma in patients 2 to 5 years of age.
Effects in Patients on Concomitant Inhaled Corticosteroids
Separate trials in adults evaluated the ability of montelukast sodium to add to the clinical effect of inhaled corticosteroids and to allow inhaled corticosteroid tapering when used concomitantly.
One randomized, placebo-controlled, parallel-group trial (n=226) enrolled adults with stable asthma with a mean FEV1 of approximately 84% of predicted who were previously maintained on various inhaled corticosteroids (delivered by metered-dose aerosol or dry powder inhalers). The median age was 41.5 years (range 16 to 70); 52.2% were females and 47.8% were males. The ethnic/racial distribution in this study was 92.0% Caucasian, 3.5% Black, 2.2% Hispanic, and 2.2% Asian. The types of inhaled corticosteroids and their mean baseline requirements included beclomethasone dipropionate (mean dose, 1203 mcg/day), triamcinolone acetonide (mean dose, 2004 mcg/day), flunisolide (mean dose, 1971 mcg/day), fluticasone propionate (mean dose, 1083 mcg/day), or budesonide (mean dose, 1192 mcg/day). Some of these inhaled corticosteroids were non-U.S.-approved formulations, and doses expressed may not be ex-actuator. The pre-study inhaled corticosteroid requirements were reduced by approximately 37% during a 5 to 7 week placebo run-in period designed to titrate patients toward their lowest effective inhaled corticosteroid dose. Treatment with montelukast sodium resulted in a further 47% reduction in mean inhaled corticosteroid dose compared with a mean reduction of 30% in the placebo group over the 12-week active treatment period (p≤0.05). It is not known whether the results of this study can be generalized to patients with asthma who require higher doses of inhaled corticosteroids or systemic corticosteroids.
In another randomized, placebo-controlled, parallel-group trial (n=642) in a similar population of adult patients previously maintained, but not adequately controlled, on inhaled corticosteroids (beclomethasone 336 mcg/day), the addition of montelukast sodium to beclomethasone resulted in statistically significant improvements in FEV1 compared with those patients who were continued on beclomethasone alone or those patients who were withdrawn from beclomethasone and treated with montelukast or placebo alone over the last 10-weeks of the 16-week, blinded treatment period. Patients who were randomized to treatment arms containing beclomethasone had statistically significantly better asthma control than those patients randomized to montelukast sodium alone or placebo alone as indicated by FEV1, daytime asthma symptoms, PEFR, nocturnal awakenings due to asthma, and “as-needed” β-agonist requirements.
In adult patients with asthma with documented aspirin sensitivity, nearly all of whom were receiving concomitant inhaled and/or oral corticosteroids, a 4-week, randomized, parallel-group trial (n=80) demonstrated that montelukast sodium, compared with placebo, resulted in significant improvement in parameters of asthma control. The magnitude of effect of montelukast sodium in aspirin-sensitive patients was similar to the effect observed in the general population of asthma patients studied. The effect of montelukast sodium on the bronchoconstrictor response to aspirin or other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients has not been evaluated [see Warnings and Precautions (5.4)].
14.3 Allergic Rhinitis (Seasonal and Perennial)
Seasonal Allergic Rhinitis
The efficacy of montelukast sodium tablets for the treatment of seasonal allergic rhinitis was investigated in 5 similarly designed, randomized, double-blind, parallel-group, placebo- and active-controlled (loratadine) trials conducted in North America. The 5 trials enrolled a total of 5029 patients, of whom 1799 were treated with montelukast sodium tablets. Patients were 15 to 82 years of age with a history of seasonal allergic rhinitis, a positive skin test to at least one relevant seasonal allergen, and active symptoms of seasonal allergic rhinitis at study entry.
The period of randomized treatment was 2 weeks in 4 trials and 4 weeks in one trial. The primary outcome variable was mean change from baseline in daytime nasal symptoms score (the average of individual scores of nasal congestion, rhinorrhea, nasal itching, sneezing) as assessed by patients on a 0 to 3 categorical scale.
Four of the five trials showed a significant reduction in daytime nasal symptoms scores with montelukast sodium tablets, 10 mg compared with placebo. The results of one trial are shown below. The median age in this trial was 35.0 years (range 15 to 81); 65.4% were females and 34.6% were males. The ethnic/racial distribution in this study was 83.1% Caucasian, 6.4% other origins, 5.8% Black, and 4.8% Hispanic. The mean changes from baseline in daytime nasal symptoms score in the treatment groups that received montelukast sodium tablets, loratadine, and placebo are shown in TABLE 9. The remaining three trials that demonstrated efficacy showed similar results. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening.
|
Treatment Group (N) |
Baseline Mean Score |
Mean Change from Baseline |
Difference Between Treatment and Placebo (95% CI) Least-Squares Mean |
|
Montelukast 10 mg (344) |
2.09 |
-0.39 |
-0.13† (-0.21, -0.06) |
|
Placebo (351) |
2.10 |
-0.26 |
N.A. |
|
Active Control‡ (Loratadine 10 mg) (599) |
2.06 |
-0.46 |
-0.24† (-0.31, -0.17) |
| * Average of individual scores of nasal congestion, rhinorrhea, nasal itching, sneezing as assessed by patients on a 0 to 3 categorical scale. † Statistically different from placebo (p≤0.001). ‡ The study was not designed for statistical comparison between montelukast sodium and the active control (loratadine). |
|||
Perennial Allergic Rhinitis
The efficacy of montelukast sodium tablets for the treatment of perennial allergic rhinitis was investigated in 2 randomized, double-blind, placebo-controlled studies conducted in North America and Europe. The two studies enrolled a total of 3357 patients, of whom 1632 received montelukast sodium tablets, 10 mg. Patients 15 to 82 years of age with perennial allergic rhinitis as confirmed by history and a positive skin test to at least one relevant perennial allergen (dust mites, animal dander, and/or mold spores), who had active symptoms at the time of study entry, were enrolled.
In the study in which efficacy was demonstrated, the median age was 35 years (range 15 to 81); 64.1% were females and 35.9% were males. The ethnic/racial distribution in this study was 83.2% Caucasian, 8.1% Black, 5.4% Hispanic, 2.3% Asian, and 1.0% other origins. Montelukast sodium tablets, 10 mg once daily was shown to significantly reduce symptoms of perennial allergic rhinitis over a 6-week treatment period (TABLE 10); in this study the primary outcome variable was mean change from baseline in daytime nasal symptoms score (the average of individual scores of nasal congestion, rhinorrhea, and sneezing).
|
Treatment Group (N) |
Baseline Mean Score |
Mean Change from Baseline |
Difference Between Treatment and Placebo (95% CI) Least-Squares Mean |
|
Montelukast 10 mg (1000) |
2.09 |
-0.42 |
-0.08† (-0.12, -0.04) |
|
Placebo (980) |
2.10 |
-0.35 |
N.A. |
| * Average of individual scores of nasal congestion, rhinorrhea, sneezing as assessed by patients on a 0 to 3 categorical scale. † Statistically different from placebo (p≤0.001). |
|||
The other 6-week study evaluated montelukast 10 mg (n=626), placebo (n=609), and an active-control (cetirizine 10 mg; n=120). The primary analysis compared the mean change from baseline in daytime nasal symptoms score for montelukast sodium vs. placebo over the first 4 weeks of treatment; the study was not designed for statistical comparison between montelukast sodium and the active-control. The primary outcome variable included nasal itching in addition to nasal congestion, rhinorrhea, and sneezing. The estimated difference between montelukast sodium and placebo was -0.04 with a 95% CI of (-0.09, 0.01). The estimated difference between the active-control and placebo was -0.10 with a 95% CI of (-0.19, -0.01).
16 HOW SUPPLIED/STORAGE AND HANDLING
Montelukast sodium oral granules USP, 4 mg (montelukast) are white to slightly yellow granules with 500 mg net weight, packed in a child-resistant foil packet. They are supplied as follows:
4 mg – unit of use carton with 30 packets (NDC 0093-7487-56).
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from moisture and light. Store in original package.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
- Advise patients about the potential risk for serious neuropsychiatric symptoms and behavioral changes with montelukast sodium use [see Warnings and Precautions (5.1)].
- Discuss the benefits and risks of montelukast sodium with patients when prescribing or continuing treatment with montelukast sodium [see Warnings and Precautions (5.1)].
- Advise patients to monitor for changes in behavior or neuropsychiatric symptoms in patients taking montelukast sodium [see Warnings and Precautions (5.1)].
- Instruct patients to discontinue montelukast sodium and contact a healthcare provider immediately if changes in behavior or thinking that are not typical for the patient occur, or if the patient develops suicidal ideation or suicidal behavior [see Warnings and Precautions (5.1)].
- Advise patients to take montelukast sodium daily as prescribed, even when they are asymptomatic, as well as during periods of worsening asthma, and to contact their physicians if their asthma is not well controlled.
- Advise patients that oral montelukast sodium is not for the treatment of acute asthma attacks. They should have appropriate short-acting inhaled β-agonist medication available to treat asthma exacerbations. Patients who have exacerbations of asthma after exercise should be instructed to have available for rescue a short-acting inhaled β-agonist.
- Advise patients to seek medical attention if short-acting inhaled bronchodilators are needed more often than usual, or if more than the maximum number of inhalations of short-acting bronchodilator treatment prescribed for a 24-hour period are needed.
- Instruct patients to continue other anti-asthma medications as prescribed unless instructed by a physician.
- Instruct patients with known aspirin sensitivity to continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast sodium [see Warnings and Precautions (5.4)].
Distributed By:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
Rev. L 5/2021
MEDICATION GUIDE
|
Montelukast (mon′′ te loo′ kast soe′ dee um) Sodium
Oral Granules |
|||
|
What is the most important information I should know about montelukast sodium oral granules? Serious mental health problems have happened in people taking montelukast sodium oral granules or even after treatment has stopped. This can happen in people with or without a history of mental health problems. Stop taking montelukast sodium oral granules and tell your healthcare provider right away if you or your child have any unusual changes in behavior or thinking, including any of these symptoms: |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
| ||
| |||
|
What are montelukast sodium oral granules? Montelukast sodium oral granules are a prescription medicine that blocks substances in the body called leukotrienes. This may help to improve symptoms of asthma and inflammation of the lining of the nose (allergic rhinitis). Montelukast sodium oral granules do not contain a steroid. Montelukast sodium oral granules are used to: 1. Prevent asthma attacks and for the long-term treatment of asthma in adults and children ages 12 months and older. Do not take montelukast sodium oral granules if you need relief right away for a sudden asthma attack. If you have an asthma attack, you should follow the instructions your healthcare provider gave you for treating asthma attacks. 2. Help control the symptoms of allergic rhinitis such as sneezing, stuffy nose, runny nose, and itching of the nose. Montelukast sodium oral granules are used to treat the following in people who have already taken other medicines that did not work well enough or in people who could not tolerate other medicines:
|
|||
|
Do not take montelukast sodium oral granules if you are allergic to any of their ingredients. See the end of this Medication Guide for a complete list of the ingredients in montelukast sodium oral granules. |
|||
|
Before taking montelukast sodium oral granules, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how montelukast sodium oral granules work, or montelukast sodium oral granules may affect how your other medicines work. |
|||
|
How should I take montelukast sodium oral granules?
For anyone who takes montelukast sodium oral granules:
For anyone 2 years of age and older with seasonal allergic rhinitis, or for anyone 6 months of age and older with perennial allergic rhinitis:
|
|||
|
What should I avoid while taking montelukast sodium oral granules? If you have asthma and aspirin makes your asthma symptoms worse, continue to avoid taking aspirin or other medicines called non-steroidal anti-inflammatory drugs (NSAIDs) while taking montelukast sodium oral granules. |
|||
|
What are the possible side effects of montelukast sodium oral granules? Montelukast sodium oral granules may cause serious side effects, including:
Tell your healthcare provider right away if you get one or more of these symptoms: |
|||
|
|
||
|
|
||
|
The most common side effects of montelukast sodium oral granules include: |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
These are not all the possible side effects of montelukast sodium oral granules. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
How should I store montelukast sodium oral granules?
|
|||
|
General information about the safe and effective use of montelukast sodium oral granules Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use montelukast sodium oral granules for a condition for which they were not prescribed. Do not give montelukast sodium oral granules to other people even if they have the same symptoms you have. They may harm them. You can ask your pharmacist or healthcare provider for information about montelukast sodium oral granules that is written for health professionals. For more information, call 1-888-838-2872. |
|||
|
What are the ingredients in montelukast sodium oral granules? Active ingredient: montelukast sodium Inactive ingredients: hydroxypropyl cellulose, magnesium stearate, mannitol, and sodium lauryl sulfate. Distributed By: Teva Pharmaceuticals USA, Inc., Parsippany, NJ 07054 |
|||
This Medication Guide has been approved by the U.S. Food and Drug Administration Rev. B 5/2021
INSTRUCTIONS FOR USE
Montelukast (mon′′ te loo′ kast soe′ dee um) Sodium
Oral Granules
This Instructions for Use contains information on how to use montelukast sodium oral granules.
Important Information:
- Before giving a dose of montelukast sodium oral granules, read this Instructions for Use to be sure you prepare and give the oral granules correctly.
- Give montelukast sodium oral granules to your child exactly as instructed by your healthcare provider.
- Stop giving montelukast sodium oral granules and tell your healthcare provider right away if your child has any unusual changes in behavior or thinking.
- Continue to give your child their asthma medicines as prescribed, unless your healthcare provider tells you to change how you give these medicines.
- You can give montelukast sodium oral granules with food or without food.
How can I give montelukast sodium oral granules to my child?
- Do not open the packet until ready to use.
- There are different ways you can give montelukast sodium 4 mg oral granules. You should choose the best method for your child:
- right into the mouth
- dissolved in 1 teaspoonful (5 mL) of cold or room temperature baby formula or breast milk
- mixed with 1 spoonful of one of the following soft foods at cold or room temperature: applesauce, mashed carrots, rice, or ice cream.
- Give the child all of the mixture within 15 minutes.
- Do not store any leftover montelukast sodium mixture (oral granules mixed with food, baby formula, or breast milk) for use at a later time. Throw away any unused portion.
- Do not mix montelukast sodium oral granules with any liquid drink other than baby formula or breast milk. Your child may drink other liquids after swallowing the mixture.
How should I store montelukast sodium oral granules?
- Store montelukast sodium oral granules at room temperature between 68° to 77°F (20° to 25°C).
- Keep montelukast sodium oral granules in the package it comes in.
- Keep montelukast sodium oral granules in a dry place and keep it away from light.
- Keep montelukast sodium oral granules and all medicines out of the reach of children.
Distributed By:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Rev. B 5/2021
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
NDC 0093-7487-56
Montelukast Sodium
Oral Granules USP, 4 mg*
*Each packet contains 4.2 mg montelukast sodium, USP equivalent to 4 mg montelukast.
For Pediatric Patients 6 months to 5 years of age.
DIRECTIONS FOR USE: See package insert for full prescribing information. Once opened, use the contents
of this packet within 15 minutes (with or without mixing with food). Discard any unused portion.
PHARMACIST: DISPENSE THE ACCOMPANYING MEDICATION GUIDE TO EACH PATIENT
Rx only
30 Packets

| MONTELUKAST SODIUM
montelukast sodium granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Teva Pharmaceuticals USA, Inc. (001627975) |