Label: SAIZEN- somatropin kit
SAIZENPREP- somatropin kit
- NDC Code(s): 44087-0016-1, 44087-1005-2, 44087-1088-1
- Packager: EMD Serono, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SAIZEN® safely and effectively. See full prescribing information for SAIZEN.
SAIZEN (somatropin) for injection, for subcutaneous use
Initial U.S. Approval: 1987INDICATIONS AND USAGE
SAIZEN is a recombinant human growth hormone indicated for:
Pediatric: Treatment of children with growth failure due to growth hormone deficiency (GHD) (1.1)
Adult: Treatment of adults with either adult onset or childhood onset GHD. (1.2)
DOSAGE AND ADMINISTRATION
- Pediatric GHD: 0.18 mg/kg/week, divided into equal doses given either on 3 alternate days, 6 times per week or daily (2.1)
-
Adult GHD: Either a non-weight based or a weight based dosing regimen may be followed, with doses adjusted based on treatment response and IGF-1 concentrations (2.2)
Non-weight-based dosing: A starting dose of approximately 0.2 mg/day (range, 0.15-0.30 mg/day) may be used without consideration of body weight, and increased gradually every 1 to 2 months by increments of approximately 0.1 to 0.2 mg/day (2.2)
Weight-based dosing: The recommended initial dose is not more than 0.005 mg/kg/day; the dose may be increased as tolerated to not more than 0.01 mg/kg/day after 4 weeks (2.2) - Injection sites should always be rotated to avoid lipoatrophy (2.3)
DOSAGE FORMS AND STRENGTHS
- SAIZEN lyophilized powder in vial (3): 5 mg and 8.8 mg
- saizenprep® reconstitution device: One vial SAIZEN containing 8.8 mg somatropin and one cartridge diluent containing 1.51 mL 0.3% (w/v) metacresol in Sterile Water for Injection
CONTRAINDICATIONS
- Acute Critical Illness (4)
- Children with Prader-Willi syndrome who are severely obese or have severe respiratory impairment – reports of sudden death (4)
- Active Malignancy (4)
- Hypersensitivity to somatropin or excipients (4)
- Active Proliferative or Severe Non-Proliferative Diabetic Retinopathy (4)
- Children with closed epiphyses (4)
WARNINGS AND PRECAUTIONS
- Acute Critical Illness: Potential benefit of treatment continuation should be weighed against the potential risk (5.1)
- Prader-Willi syndrome in Children: Evaluate for signs of upper airway obstruction and sleep apnea before initiation of treatment. Discontinue treatment if these signs occur (5.2)
- Neoplasms: Monitor patients with preexisting tumors for progression or recurrence. Increased risk of a second neoplasm in childhood cancer survivors treated with somatropin—in particular meningiomas as in patients treated with radiation to the head for their first neoplasm (5.3)
- Impaired Glucose Tolerance and Diabetes Mellitus: May be unmasked. Periodically monitor glucose levels in all patients Doses of concurrent antihyperglycemic drugs in diabetics may require adjustment (5.4)
- Intracranial Hypertension: Exclude preexisting papilledema. May develop and is usually reversible after discontinuation or dose reduction (5.5)
- Hypersensitivity: Serious hypersensitivity reactions may occur. In the event of an allergic reaction, seek prompt medical attention (5.6)
- Fluid Retention (i.e., edema, arthralgia, carpal tunnel syndrome – especially in adults): May occur frequently. Reduce dose as necessary (5.7)
- Hypoadrenalism: Monitor patients for reduced serum cortisol levels and/or need for glucocorticoid dose increases in those with known hypoadrenalism (5.8)
- Hypothyroidism: May first become evident or worsen (5.9)
- Slipped Capital Femoral Epiphysis: May develop. Evaluate children with the onset of a limp or hip/knee pain (5.10)
- Progression of Preexisting Scoliosis: May develop (5.11)
- Reevaluation of Childhood Onset Adult GHD (5.12)
- Pancreatitis: Consider pancreatitis in patients with persistent severe abdominal pain (5.15)
- Benzyl Alcohol (5.16)
ADVERSE REACTIONS
Most common adverse reactions are injection site reactions (such as pain, numbness, redness, and swelling), fluid retention, peripheral edema, arthralgia, myalgia, paresthesia, and headache. (6)
To report SUSPECTED ADVERSE REACTIONS, contact EMD Serono at 1-800-283-8088 ext 5563 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Inhibition of 11ß-Hydroxysteroid Dehydrogenase Type 1: May require the initiation of glucocorticoid replacement therapy. Patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance doses (7.1)
- Glucocorticoid Replacement: Should be carefully adjusted (7.2)
- Cytochrome P450-Metabolized Drugs: Monitor carefully if used with somatropin (7.3)
- Oral Estrogen: Larger doses of somatropin may be required in women (7.4)
- Insulin and/or Oral/Injectable Hypoglycemic Agents: May require adjustment (7.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pediatric Patients
1.2 Adult Patients
2 DOSAGE AND ADMINISTRATION
2.1 Pediatric Growth Hormone Deficiency (GHD)
2.2 Adult Growth Hormone Deficiency (GHD)
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Critical Illness
5.2 Prader-Willi Syndrome in Children
5.3 Neoplasms
5.4 Glucose Intolerance/Diabetes Mellitus
5.5 Intracranial Hypertension
5.6 Severe Hypersensitivity
5.7 Fluid Retention
5.8 Hypoadrenalism
5.9 Hypothyroidism
5.10 Slipped Capital Femoral Epiphysis in Pediatric Patients
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
5.12 Reevaluation of Childhood Onset Adult GHD
5.13 Lipoatrophy
5.14 Laboratory Tests
5.15 Pancreatitis
5.16 Benzyl Alcohol
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Inhibition of 11β-Hydroxysteroid Dehydrogenase Type 1 (11βHSD-1)
7.2 Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment
7.3 Cytochrome P450-Metabolized Drugs
7.4 Oral Estrogen
7.5 Insulin and/or Oral/Injectable Hypoglycemic Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Gender Effect
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pediatric Patients
SAIZEN (somatropin) is indicated for the treatment of pediatric patients with growth failure due to inadequate secretion of endogenous growth hormone.
1.2 Adult Patients
SAIZEN is indicated for replacement of endogenous growth hormone in adults with growth hormone deficiency who meet either of the following two criteria:
Adult Onset
Patients who have growth hormone deficiency, either alone or associated with multiple hormone deficiencies (hypopituitarism), as a result of pituitary disease, hypothalamic disease, surgery, radiation therapy, or trauma; or
Childhood Onset
Patients who were growth hormone deficient during childhood as a result of congenital, genetic, acquired, or idiopathic causes.
Patients who were treated with somatropin for growth hormone deficiency in childhood and whose epiphyses are closed should be reevaluated before continuation of somatropin therapy at the reduced dose level recommended for growth hormone deficient adults. Confirmation of the diagnosis of adult growth hormone deficiency in both groups involves an appropriate growth hormone provocative test with two exceptions: (1) patients with multiple other pituitary hormone deficiencies due to organic disease; and (2) patients with congenital/genetic growth hormone deficiency.
-
2 DOSAGE AND ADMINISTRATION
For subcutaneous injection.
SAIZEN therapy should be supervised by a physician who is experienced in the diagnosis and management of pediatric patients with growth hormone deficiency or adult patients with either childhoodonset or adult-onset growth hormone deficiency.
2.1 Pediatric Growth Hormone Deficiency (GHD)
SAIZEN dosage and administration schedule should be individualized for each patient. The recommended weekly dosage is 0.18 mg/kg of body weight by subcutaneous injection. It should be divided into equal doses given either on 3 alternate days, 6 times per week or daily.
Response to somatropin therapy in pediatric patients tends to decrease with time. However, in pediatric patients, the failure to increase growth rate, particularly during the first year of therapy, indicates the need for close assessment of compliance and evaluation for other causes of growth failure, such as hypothyroidism, undernutrition, advanced bone age and antibodies to recombinant human growth hormone.
Treatment with SAIZEN of growth failure due to growth hormone deficiency should be discontinued when the epiphyses are fused.
2.2 Adult Growth Hormone Deficiency (GHD)
Either of two approaches to SAIZEN dosing may be followed: a weight-based regimen or a non-weight-based regimen.
Weight-based
Based on the dosing utilized in the original pivotal study described herein, the recommended dosage at the start of therapy is not more than 0.005 mg/kg given as a daily subcutaneous injection. The dosage may be increased to not more than 0.01 mg/kg/day after 4 weeks according to individual patient requirements. Clinical response, side effects, and determination of age-and gender-adjusted serum insulin-like growth factor (IGF-1) levels may be used as guidance in dose titration.
Non-weight-based
Alternatively, taking into account more recent literature, a starting dose of approximately 0.2 mg/day (range, 0.15-0.30 mg/day) may be used without consideration of body weight. This dose can be increased gradually every 1 to 2 months by increments of approximately 0.1 to 0.2 mg/day, according to individual patient requirements based on the clinical response and serum IGF-1 concentrations. During therapy, the dose should be decreased if required by the occurrence of adverse reactions and/or serum IGF-1 levels above the age- and gender-specific normal range. Maintenance dosages vary considerably from person to person.
A lower starting dose and smaller dose increments should be considered for older patients, who are more prone to the adverse effects of somatropin than younger individuals. In addition, obese individuals are more likely to manifest adverse effects when treated with a weight-based regimen. In order to reach the defined treatment goal, estrogen-replete women may need higher doses than men. Oral estrogen administration may increase the dose requirements in women.
2.3 Preparation and Administration
Prior to self-administration of the product at home, ensure to train patients and caregivers how to prepare and administer the product correctly to help avoid wrong technique and dosing errors.
Vials
To prevent possible contamination, wipe the rubber vial stopper with an antiseptic solution before puncturing it with the needle. It is recommended that SAIZEN be administered using sterile, disposable syringes and needles. The syringes should be of small enough volume that the prescribed dose can be drawn from the vial with reasonable accuracy.
After determining the appropriate patient dose, reconstitute each vial of SAIZEN as follows: 5 mg vial with 1 to 3 mL of Bacteriostatic Water for Injection, USP (Benzyl Alcohol preserved); 8.8 mg vial with 2-3 mL of Bacteriostatic Water for Injection, USP (Benzyl Alcohol preserved). Approximately 10% mechanical loss can be associated with reconstitution and multidose administration.
If sensitivity to the diluent occurs, SAIZEN may be reconstituted with Sterile Water for Injection, USP. When SAIZEN is reconstituted in this manner, the reconstituted solution should be used immediately and any unused solution should be discarded [see Warnings and Precautions (5.15)].
To reconstitute SAIZEN, inject the diluent into the vial of SAIZEN aiming the liquid against the glass vial wall. Swirl the vial with a GENTLE rotary motion until contents are dissolved completely. DO NOT SHAKE. Parenteral drug products should always be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. SAIZEN MUST NOT BE INJECTED if the solution is cloudy or contains particulate matter. Use it only if it is clear and colorless.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
• Acute Critical Illness
Treatment with pharmacologic amounts of somatropin is contraindicated in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure [see Warnings and Precautions (5.1)].
• Prader-Willi Syndrome in Children
Somatropin is contraindicated in patients with Prader-Willi syndrome who are severely obese or have severe respiratory impairment [see Warnings and Precautions (5.2)]. There have been reports of sudden death when somatropin was used in such patients. SAIZEN is not indicated for the long term treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
• Active Malignancy
In general, somatropin is contraindicated in the presence of active malignancy. Any pre-existing malignancy should be inactive and its treatment complete prior to instituting therapy with somatropin. Somatropin should be discontinued if there is evidence of recurrent activity. Since growth hormone deficiency may be an early sign of the presence of a pituitary tumor (or, rarely, other brain tumors), the presence of such tumors should be ruled out prior to initiation of treatment. Somatropin should not be used in patients with any evidence of progression or recurrence of an underlying intracranial tumor [See Warnings and Precautions (5.3)].
• Hypersensitivity
SAIZEN is contraindicated in patients with a known hypersensitivity to somatropin or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products [see Warnings and Precautions (5.6)].
• Diabetic Retinopathy
Somatropin is contraindicated in patients with active proliferative or severe non-proliferative diabetic retinopathy.
• Closed Epiphyses
Somatropin should not be used for growth promotion in pediatric patients with closed epiphyses.
• Benzyl Alcohol
SAIZEN reconstituted with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) should not be administered to patients with a known sensitivity to Benzyl Alcohol [see Warnings and Precautions (5.16)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Critical Illness
Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported after treatment with pharmacologic amounts of somatropin [see Contraindications (4)]. Two placebo-controlled clinical trials in non-growth hormone deficient adult patients (n=522) with these conditions in intensive care units revealed a significant increase in mortality (42% vs. 19%) among somatropin-treated patients (doses 5.3-8 mg/day) compared to those receiving placebo. The safety of continuing somatropin treatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. Therefore, the potential benefit of treatment continuation with somatropin in patients having acute critical illnesses should be weighed against the potential risk.
5.2 Prader-Willi Syndrome in Children
There have been reports of fatalities after initiating therapy with somatropin in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. Patients with Prader-Willi syndrome should be evaluated for signs of upper airway obstruction and sleep apnea before initiation of treatment with somatropin. If, during treatment with somatropin, patients show signs of upper airway obstruction (including onset of or increased snoring) and/or new onset sleep apnea, treatment should be interrupted. All patients with Prader-Willi syndrome treated with somatropin should also have effective weight control and be monitored for signs of respiratory infection, which should be diagnosed as early as possible and treated aggressively [see Contraindications (4)]. SAIZEN is not indicated for the long term treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
5.3 Neoplasms
In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm has been reported. Intracranial tumors, in particular meningiomas, were the most common of these second neoplasms. In adults, it is unknown whether there is any relationship between somatropin replacement therapy and CNS tumor recurrence [see Contraindications (4)]. Monitor all patients with a history of GHD secondary to an intracranial neoplasm routinely while on somatropin therapy for progression or recurrence of the tumor.
Because children with certain rare genetic causes of short stature have an increased risk of developing malignancies, practitioners should thoroughly consider the risks and benefits of starting somatropin in these patients. If treatment with somatropin is initiated, these patients should be monitored carefully for development of neoplasms.
Monitor patients on somatropin therapy carefully for increased growth, or potential malignant changes of preexisting nevi.
5.4 Glucose Intolerance/Diabetes Mellitus
Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses in susceptible patients. As a result, previously undiagnosed impaired glucose tolerance and overt diabetes mellitus may be unmasked during somatropin treatment and new onset type 2 diabetes mellitus has been reported in patients. Therefore, glucose levels should be monitored periodically in all patients treated with somatropin, especially in those with risk factors for diabetes mellitus, such as obesity, Turner syndrome, or a family history of diabetes mellitus. Patients with preexisting type 1 or type 2 diabetes mellitus or impaired glucose tolerance should be monitored closely during somatropin therapy. The doses of antihyperglycemic drugs (i.e., insulin or oral agents) may require adjustment when somatropin therapy is instituted in these patients.
5.5 Intracranial Hypertension
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea, and/or vomiting has been reported in a small number of patients treated with somatropin products. Symptoms usually occurred within the first eight (8) weeks after the initiation of somatropin therapy. In all reported cases, IH-associated signs and symptoms rapidly resolved after cessation of therapy or a reduction of the somatropin dose. Funduscopic examination should be performed routinely before initiating treatment with somatropin to exclude preexisting papilledema, and periodically during the course of somatropin therapy. If papilledema is observed by funduscopy during somatropin treatment, treatment should be stopped. If somatropin-induced IH is diagnosed, treatment with somatropin can be restarted at a lower dose after IH-associated signs and symptoms have resolved. Patients with Turner syndrome, chronic renal insufficiency, and Prader-Willi syndrome may be at increased risk for the development of IH.
5.6 Severe Hypersensitivity
Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products. Patients and caregivers should be informed that such reactions are possible and that prompt medical attention should be sought if an allergic reaction occurs [see Contraindications (4)].
5.7 Fluid Retention
Fluid retention during somatropin replacement therapy in adults may occur. Clinical manifestations of fluid retention (e.g. edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) are usually transient and dose dependent.
5.8 Hypoadrenalism
Patients receiving somatropin therapy who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of somatropin treatment [see Section 7.1, 11-β Hydroxysteroid Dehydrogenase Type 1].
5.9 Hypothyroidism
Undiagnosed/untreated hypothyroidism may prevent an optimal response to somatropin, in particular, the growth response in children. Patients with Turner syndrome have an inherently increased risk of developing autoimmune thyroid disease and primary hypothyroidism. In patients with growth hormone deficiency, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Therefore, patients treated with somatropin should have periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or appropriately adjusted when indicated.
5.10 Slipped Capital Femoral Epiphysis in Pediatric Patients
Slipped capital femoral epiphysis may occur more frequently in patients with endocrine disorders (including pediatric growth hormone deficiency and Turner syndrome) or in patients undergoing rapid growth. Any pediatric patient with the onset of a limp or complaints of hip or knee pain during somatropin therapy should be carefully evaluated.
5.11 Progression of Preexisting Scoliosis in Pediatric Patients
Progression of scoliosis can occur in patients who experience rapid growth. Because somatropin increases growth rate, patients with a history of scoliosis who are treated with somatropin should be monitored for progression of scoliosis. However, somatropin has not been shown to increase the occurrence of scoliosis. Skeletal abnormalities including scoliosis are commonly seen in untreated Turner syndrome patients. Scoliosis is also commonly seen in untreated patients with Prader-Willi syndrome. Physicians should be alert to these abnormalities, which may manifest during somatropin therapy.
5.12 Reevaluation of Childhood Onset Adult GHD
Patients with epiphyseal closure who were treated with somatropin replacement therapy in childhood should be reevaluated according to the criteria in INDICATIONS AND USAGE before continuation of somatropin therapy at the reduced dose level recommended for growth hormone deficient adults. Experience with prolonged treatment in adults is limited.
5.13 Lipoatrophy
When somatropin is administered subcutaneously at the same site over a long period of time, tissue atrophy may result. This can be avoided by rotating the injection site [see Dosage and Administration (2.3)].
5.14 Laboratory Tests
Serum levels of inorganic phosphorus, alkaline phosphatase, parathyroid hormone (PTH), and IGF-1 may increase with somatropin therapy.
5.15 Pancreatitis
Cases of pancreatitis have been reported rarely in children and adults receiving somatropin treatment, with some evidence supporting a greater risk in children compared with adults. Published literature indicates that girls who have Turner syndrome may be at greater risk than other somatropin-treated children. Pancreatitis should be considered in any somatropin–treated patient, especially a child, who develops persistent severe abdominal pain.
5.16 Benzyl Alcohol
Benzyl alcohol, a component of this product, has been associated with serious adverse events and death, particularly in pediatric patients. The "gasping syndrome," (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low-birth weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
-
6 ADVERSE REACTIONS
The following important adverse reactions are also described elsewhere in the labeling:
- Increased mortality in patients with acute critical illness [see Warnings and Precautions (5.1)]
- Fatalities in children with Prader-Willi syndrome [see Warnings and Precautions (5.2)]
- Neoplasms [see Warnings and Precautions (5.3)]
- Glucose intolerance and diabetes mellitus [see Warnings and Precautions (5.4)]
- Intracranial hypertension [see Warnings and Precautions (5.5)]
- Severe hypersensitivity [see Warnings and Precautions (5.6)]
- Fluid retention [see Warnings and Precautions (5.7)]
- Hypoadrenalism [see Warnings and Precautions (5.8)
- Hypothyroidism [see Warnings and Precautions (5.9)]
- Slipped capital femoral epiphysis in pediatric patients [see Warnings and Precautions (5.10)]
- Progression of preexisting scoliosis in pediatric patients [see Warnings and Precautions (5.11)]
- Lipoatrophy [see Warnings and Precautions (5.13)]
- Pancreatitis [see Warnings and Precautions (5.15)]
- Benzyl alcohol [see Warnings and Precautions (5.16)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed during the clinical trials performed with one somatropin formulation cannot always be directly compared to the rates observed during the clinical trials performed with a second somatropin formulation, and may not reflect the adverse reaction rates observed in practice.
Growth Hormone Deficient Pediatric Patients
In clinical studies in which SAIZEN was administered to growth hormone deficient children, the following reactions were infrequently seen: local reactions at the injection site (such as pain, numbness, redness and swelling), hypothyroidism, hypoglycemia, seizures, exacerbation of preexisting psoriasis and disturbances in fluid balance.
Growth Hormone Deficient Adult Patients
For a description of the clinical trials refer to section 14. During the 6-month placebo-controlled study, adverse reactions were reported in 56 patients (93.3%) in the somatropin-treated group and 42 patients (76.4%) in the placebo-treated group. Adverse reactions with an incidence of ≥5% in SAIZEN-treated patients which were more frequent in SAIZEN-treated patients compared with placebo-treated patients are listed in Table 1. Arthralgia, myalgia, peripheral edema, other types of edema, carpal tunnel syndrome, paraesthesia and hypoaesthesia were common in the somatropin-treated patients and reported more frequently than in the placebo group. These types of adverse reactions are thought to be related to the fluid accumulating effects of somatropin. During the placebo-controlled portion of the study, approximately 10% of patients without preexisting diabetes mellitus or impaired glucose tolerance treated with somatropin manifested mild, but persistent, abnormalities of glucose tolerance, compared with none in the placebo group. During the open label phase of the study, approximately 10% of patients treated with somatropin required a small upward adjustment of thyroid hormone replacement therapy for preexisting central hypothyroidism and 1 patient was newly diagnosed with central hypothyroidism. In addition, during the open label phase of the study, when all patients were being treated with somatropin, two patients with preexisting central hypoadrenalism required upward titration of hydrocortisone maintenance therapy which was considered to be suboptimal (unrelated to intercurrent stress, surgery or disease), and 1 patient was diagnosed de novo with central adrenal insufficiency after six months of somatropin treatment. Anti-GH antibodies were not detected.
Table 1 Adverse Reactions with ≥5% Overall Incidence in SAIZEN-Treated Patients Which Were More Frequent in SAIZEN-Treated Patients Compared with Placebo-Treated Patients During a 6 Month Study Adverse Reaction SAIZEN-Treated (N=60) Placebo (N=55) N = number of patients Arthralgia 14(23.3%) 7(12.7%) Headache 11(18.3%) 8(14.5%) Edema peripheral 9(15.0%) 2(3.7%) Myalgia 5(8.3%) 2(3.6%) Paraesthesia 4(6.7%) 1(1.8%) Hypoaesthesia 4(6.7%) 0 Edema dependent 3(5.0%) 2(3.6%) Skeletal Pain 3(5.0%) 1(1.8%) Carpal tunnel syndrome 3(5.0%) 1(1.8%) Edema generalized 3(5.0%) 0 Chest pain 3(5.0%) 0 Depression 3(5.0%) 0 Hypothyroidism 3(5.0%) 0 Insomnia 3(5.0%) 0 The adverse reaction pattern observed during the open label phase of the study was similar to the one presented above.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to SAIZEN with the incidence of antibodies to other products may be misleading. In the case of growth hormone, antibodies with binding capacities lower than 2 mg/mL have not been associated with growth attenuation. In a very small number of patients treated with somatropin, when binding capacity was greater than 2 mg/mL, interference with the growth response was observed.
6.3 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of SAIZEN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products [See Warnings and Precautions (5.6)].
Leukemia has been reported in a small number of growth hormone deficient patients treated with growth hormone. It is uncertain whether this increased risk is related to the pathology of growth hormone deficiency itself, growth hormone therapy, or other associated treatments such as radiation therapy for intracranial tumors. So far, epidemiological data fail to confirm the hypothesis of a relationship between growth hormone therapy and leukemia.
The following additional adverse reactions have been observed during the appropriate use of somatropin: headaches (children and adults), gynecomastia (children), and pancreatitis (children and adults) (see Warnings and Precautions [5.14]).
-
7 DRUG INTERACTIONS
7.1 Inhibition of 11β-Hydroxysteroid Dehydrogenase Type 1 (11βHSD-1)
The microsomal enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) is required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. GH and somatropin inhibit 11βHSD-1. Consequently, individuals with untreated GH deficiency have relative increases in 11βHSD-1 and serum cortisol. Introduction of somatropin treatment may result in inhibition of 11βHSD-1 and reduced serum cortisol concentrations. As a consequence, previously undiagnosed central (secondary) hypoadrenalism may be unmasked and glucocorticoid replacement may be required in patients treated with somatropin. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of somatropin treatment; this may be especially true for patients treated with cortisone acetate and prednisone since conversion of these drugs to their biologically active metabolites is dependent on the activity of 11βHSD-1 [see Warnings and Precautions (5.8)].
7.2 Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment
Pharmacologic glucocorticoid therapy and supraphysiologic glucocorticoid treatment may attenuate the growth promoting effects of somatropin in children. Therefore, glucocorticoid replacement dosing should be carefully adjusted in children receiving concomitant somatropin and glucocorticoid treatments to avoid both hypoadrenalism and an inhibitory effect on growth.
7.3 Cytochrome P450-Metabolized Drugs
Limited published data indicate that somatropin treatment increases cytochrome P450 (CYP450) mediated antipyrine clearance in man. These data suggest that somatropin administration may alter the clearance of compounds known to be metabolized by CYP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporine). Careful monitoring is advisable when somatropin is administered in combination with other drugs known to be metabolized by CYP450 liver enzymes. However, formal drug interaction studies have not been conducted.
7.4 Oral Estrogen
Because oral estrogens may reduce the serum IGF-1 response to somatropin treatment, girls and women receiving oral estrogen replacement may require greater somatropin dosages [see Dosage and Administration (2.2)].
7.5 Insulin and/or Oral/Injectable Hypoglycemic Agents
In patients with diabetes mellitus requiring drug therapy, the dose of insulin and/or oral/injectable agent may require adjustment when somatropin therapy is initiated [see Warnings and Precautions (5.4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Reproduction studies have been performed in rats and rabbits at doses up to 31 and 62 times, respectively, the human (child) weekly dose based on body surface area. The results have revealed no evidence of impaired fertility or harm to the fetus due to SAIZEN. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether SAIZEN is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SAIZEN is administered to a nursing woman.
8.5 Geriatric Use
The safety and effectiveness of SAIZEN in patients aged 65 and over has not been evaluated in clinical studies. Elderly patients may be more sensitive to the action of SAIZEN, and therefore may be more prone to develop adverse reactions. A lower starting dose and smaller dose increments should be considered for older patients [see Dosage and Administration (2.2)].
8.6 Hepatic Impairment
A reduction in somatropin clearance has been noted in patients with hepatic dysfunction as compared with normal controls. However, no studies have been conducted for SAIZEN in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Subjects with chronic renal failure tend to have decreased clearance of somatropin compared to those with normal renal function. However, no studies have been conducted for SAIZEN in patients with renal impairment [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
SAIZEN is a human growth hormone produced by recombinant DNA technology. SAIZEN has 191 amino acid residues and a molecular weight of 22,125 daltons. Its amino acid sequence and structure are identical to the dominant form of human pituitary growth hormone. SAIZEN is produced by a mammalian cell line (mouse C127) that has been modified by the addition of the human growth hormone gene.
SAIZEN is a sterile, non pyrogenic, white, lyophilized powder intended for subcutaneous injection after reconstitution with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol). The reconstituted solution has a pH of 6.5 to 8.5.
Vials
SAIZEN is available in 5 mg and 8.8 mg vials. The quantitative composition per vial is:
5 mg vial:
Each vial contains 5.0 mg somatropin, 34.2 mg sucrose and 1.16 mg O-phosphoric acid. The pH is adjusted with sodium hydroxide or O-phosphoric acid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Somatropin (as well as endogenous growth hormone) binds to dimeric growth hormone receptors located within the cell membranes of target tissue cells resulting in intracellular signal transduction and a host of pharmacodynamic effects. Some of these pharmacodynamic effects are primarily mediated by IGF-1 produced in the liver and also locally (e.g., skeletal growth, protein synthesis), while others are primarily a consequence of the direct effects of somatropin (e.g., lipolysis) [see Pharmacodynamics (12.2)].
12.2 Pharmacodynamics
Tissue Growth
Skeletal Growth: SAIZEN stimulates skeletal growth in prepubertal children with pituitary growth hormone deficiency. Skeletal growth is accomplished at the epiphyseal plates at the ends of long bone. Growth and metabolism of epiphyseal plate cells are directly stimulated by growth hormone and one of its mediators, insulin-like growth factor-1. Serum levels of insulin-like growth factor-1 (IGF-1) are low in children and adolescents who are growth hormone deficient, but increase during treatment with SAIZEN. Linear growth continues until the growth plates fuse at the end of puberty.
Protein Metabolism
Linear growth is facilitated in part by increased cellular protein synthesis. This is reflected by increased cellular uptake of amino acids and nitrogen retention as demonstrated by a decline in urinary nitrogen excretion and blood urea nitrogen during somatropin therapy.
Carbohydrate Metabolism
Somatropin is a modulator of carbohydrate metabolism. Children with inadequate secretion of growth hormone sometimes experience fasting hypoglycemia that is improved by treatment with somatropin. SAIZEN therapy may decrease glucose tolerance. Administration of SAIZEN to normal adults and patients with growth hormone deficiency resulted in transient increases in mean serum fasting and postprandial insulin levels. However, glucose levels remained in the normal range.
Lipid Metabolism
Acute administration of somatropin to humans results in lipid mobilization. Nonesterified fatty acids increase in plasma within one hour of SAIZEN administration. In growth hormone deficient patients, long-term somatropin administration often decreases body fat. Mean cholesterol levels decreased in patients treated with SAIZEN. The clinical significance of this decrease in cholesterol level is unknown.
12.3 Pharmacokinetics
Absorption - The absolute bioavailability of somatropin after subcutaneous administration ranges between 70 to 90%.
Distribution - The steady-state volume of distribution (mean ±SD) of somatropin following intravenous administration in healthy volunteers was estimated to be 12.0 ± 1.08 L.
Metabolism - The metabolic fate of somatropin involves classical protein catabolism in both the liver and kidneys. In renal cells, at least a portion of the breakdown products is returned to the systemic circulation. The mean half-life of intravenous somatropin in normal males is around 0.6 hours, whereas subcutaneously and intramuscularly administered somatropin has a half-life of around 2 hours. The longer half-life observed after subcutaneous or intramuscular administration is due to slow absorption from the injection site.
Excretion - The clearance (mean ±SD) of intravenously administered somatropin in six normal male volunteers was 14.6 ± 2.8 L/hr.
Specific Populations
Pediatric - The pharmacokinetics of somatropin is similar in children and adults. However, no pharmacokinetic studies of SAIZEN have been conducted in pediatric patients.
Gender - No gender studies have been performed in children for somatropin. In adults, the clearance of somatropin in both men and women tends to be similar. However, no studies have been conducted to evaluate the effect of gender on pharmacokinetics of SAIZEN.
Race - No studies have been conducted to determine the effect of race on the pharmacokinetics of SAIZEN.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Adult Growth Hormone Deficiency (GHD)
A multicenter, randomized, double-blind, placebo-controlled clinical trial was conducted in 115 adults with growth hormone deficiency comparing the effects of SAIZEN and placebo on body composition. Patients in the active treatment arm were treated with SAIZEN at an initial dose of 0.005 mg/kg/day for one month which was increased to 0.01 mg/kg/day if tolerated for the remaining five months of the study. The primary endpoint was the change from baseline in lean body mass measured by dual energy X-ray absorptiometry (DXA) after 6 months. Treatment with SAIZEN produced significant (p<0.001) increases from baseline in LBM compared to placebo (Table 2).
Table 2 – Lean Body Mass (kg) by DXA SAIZEN
(n=52)Placebo
(n=51)Baseline (mean) 47.7 54.0 Change from baseline at 6 months (mean) +1.9 -0.2 Treatment difference (mean)
95% confidence interval
p-value2.1
(1.3, 2.9)
<0.001Sixty-seven (58%) of the 115 randomized patients were male. The adjusted mean treatment difference on the increase in lean body mass from baseline was significantly greater in males (2.9 kg) than females (0.8 kg).
Ninety-seven (84%) of the 115 randomized patients had adult onset GHD. The adjusted mean treatment differences on the increase in lean body mass from baseline were not significantly different in AO GHD (2.1 kg) compared with childhood onset GHD (1.0 kg) patients. However, there were relatively few patients with childhood onset GHD (n=18) on which to base the comparison.
Analysis of the treatment difference on the change from baseline in total fat mass (by DXA) revealed a significant decrease (p<0.001) in the SAIZEN-treated group compared to the placebo group. SAIZEN also produced beneficial effects on several bone turnover markers including bone specific alkaline phosphatase, C-terminal propeptide, osteocalcin, urine deoxypyridinoline and iPTH.
One hundred and eleven patients were enrolled in an open label follow up study and treated with SAIZEN for an additional 6-30 months. During this period, the beneficial effects on lean body mass and total fat mass achieved during the initial six months of treatment were maintained.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SAIZEN can be administered using (1) a standard sterile disposable syringe and needle, (2) a compatible SAIZEN needle-free injection device or (3) a compatible SAIZEN needle injection device. For proper use, refer to the Instructions for Use provided with the administration device.
SAIZEN is a sterile, non pyrogenic, white, lyophilized powder supplied in packages containing:
1 vial of 5 mg SAIZEN and 1 vial of Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) NDC 44087-1005-2
1 vial of 8.8 mg SAIZEN and 1 vial of Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) NDC 44087-1088-1
1 saizenprep® cartridge of 8.8 mg SAIZEN and 1.51 mL Sterile Water for Injection 0.3% (w/v) metacresol as antimicrobial preservative NDC 44087-0016-1
16.2 Storage and Handling
Before Reconstitution - SAIZEN should be stored at room temperature (15°-30°C/59°-86°F). Expiration dates are stated on the labels.
After Reconstitution - SAIZEN 5 mg and 8.8 mg vials reconstituted with the Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol) provided should be stored under refrigeration (2°–8°C/36°–46°F) for up to 14 days.
SAIZEN 8.8 mg saizenprep® cartridge reconstituted with the Sterile Water for Injection, 0.3% (w/v) metacresol provided should be stored under refrigeration (2°-8°C/36°-46°F) for up to 21 days.
Avoid freezing reconstituted vials or cartridges of SAIZEN.
-
17 PATIENT COUNSELING INFORMATION
Prior to self-administration of the product at home, ensure to train patients and caregivers how to prepare and administer the product correctly to help avoid wrong technique and dosing errors.
Patients being treated with SAIZEN (and/or their parents) should be informed about the potential benefits and risks associated with SAIZEN treatment. This information is intended to better educate patients (and caregivers); it is not a disclosure of all possible adverse or intended effects.
Patients and caregivers who will administer SAIZEN should receive appropriate training and instruction on the proper use of SAIZEN from the physician or other suitably qualified health care professional. A puncture-resistant container for the disposal of used syringes and needles should be strongly recommended. Patients and/or parents should be thoroughly instructed in the importance of proper disposal, and cautioned against any reuse of needles and syringes. This information is intended to aid in the safe and effective administration of the medication.
To reconstitute SAIZEN, inject the diluent into the vial of SAIZEN aiming the liquid against the glass vial wall. Swirl the vial with a GENTLE rotary motion until contents are dissolved completely. DO NOT SHAKE. Parenteral drug products should always be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. SAIZEN MUST NOT BE INJECTED if the solution is cloudy or contains particulate matter. Use it only if it is clear and colorless.
Never Share a SAIZEN Needle Between Patients
Counsel patients that they should never share a SAIZEN needle with another person, even if the needle is changed. Sharing of the pen between patients may pose a risk of transmission of infection.
For drug preparation instructions for saizenprep® cartridges, please refer to the Instructions for Use provided with saizenprep® reconstitution device.
- SPL UNCLASSIFIED SECTION
-
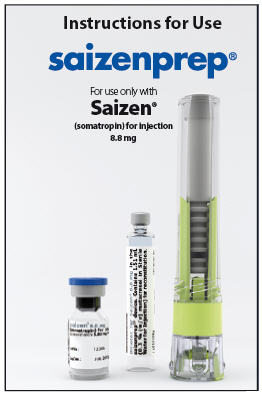
Instructions for Use
saizenprep®
For use only with
Saizen®
(somatropin) for injection
8.8 mg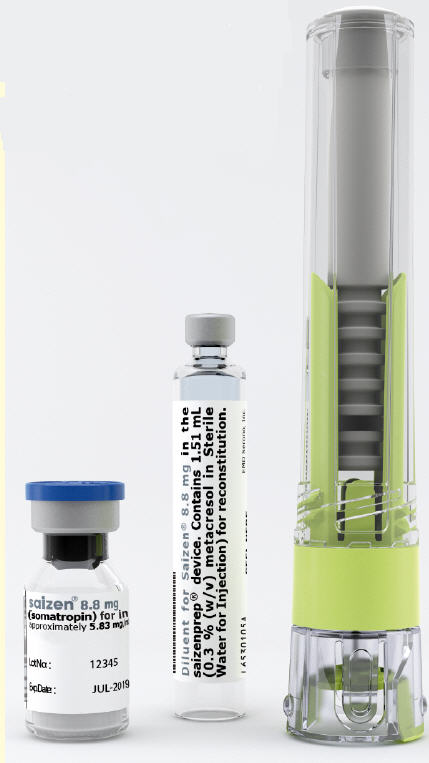
Important information
You must have received training before using saizenprep® (si-zin-prep).
Always follow all directions and training provided by your healthcare provider.
For children, use of the saizenprep® device must be supervised by an adult.
Contact our patient support services at 1-800-582-7989 for questions related to the device or Saizen® (somatropin) for injection.
What is in the box? †
Tray
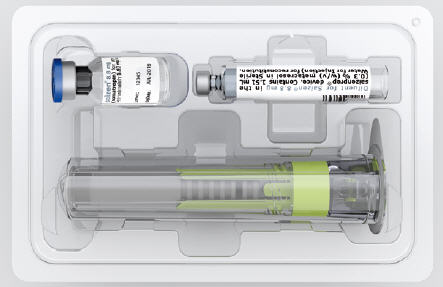
Patient Information Leaflet
saizenprep® Instructions for Use
† You will also need: sharps container, pen and alcohol wipes (not included) Important information
What is saizenprep®?
saizenprep® is a single use reconstitution (mixing) device used to mix SAIZEN.
How supplied
saizenprep® is supplied in a kit.
saizenprep® 8.8 mg contains:
8.8 mg vial and 1.51 mL cartridge.Getting to know saizenprep®
saizenprep® Reconstitution Device Diluent Cartridge 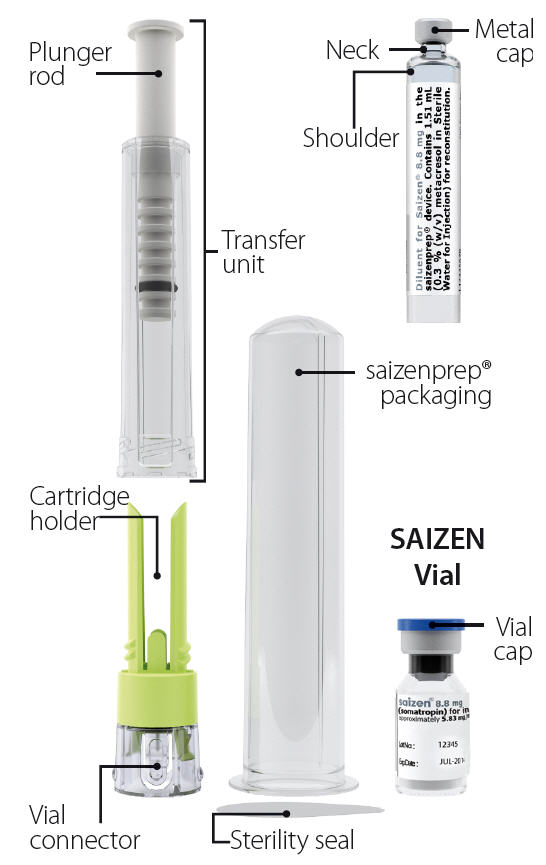
STEP 1
Check supplies
Note: Prepare a clean, hard, flat surface as a working area.
- 1.1 Box:
-
- □
- Confirm product is not expired
- □
- Open box
- □
- Carefully remove contents
- 1.2 Wash Hands:
-
- Important
- Wash Hands
- Warning: Using the device with unclean hands may lead to contamination and possibly infection.
- 1.1
-
- 1.2
-

- 1.3 SAIZEN Vial:
-
- □
- Confirm vial label says SAIZEN
- □
- Confirm product is not expired
- □
- Confirm correct strength (e.g. 8.8 mg)
- □
- Confirm vial is not cracked and cap is not damaged
- 1.4 Diluent Cartridge:
-
- □
- Confirm cartridge label says Diluent for Saizen®
- □
- Confirm product is not expired
- □
- Confirm correct volume (e.g. 1.51 mL)
- □
- Confirm cartridge is not cracked
- 1.3 Vial
-
- 1.4 Cartridge
-
- 1.5 saizenprep® Packaging and Sterility Seal:
-
- □
- Confirm product is not expired
- □
- Confirm sterility seal is not damaged or loose
- □
- Confirm packaging is not cracked or damaged
Warning: Do not use if any items are damaged or expired. Call patient support services at 1-800-582-7989 for assistance.
- 1.5
-

STEP 2
Prepare saizenprep®
SAIZEN Vial:
- 2.1
- Remove blue protective flip off vial cap from vial and throw away (discard) in the trash.
- 2.2
- Clean gray rubber at top of vial with alcohol wipe.
- 2.1
-

- 2.2
-
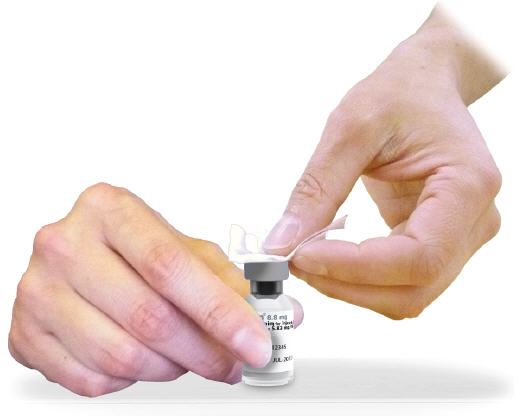
Diluent Cartridge:
- 2.3
- Grasp arrow tab to peel outer label and tear off at perforation before discarding in the trash.
- 2.4
- Clean rubber at top of cartridge with alcohol wipe.
- 2.3
-

- 2.4
-

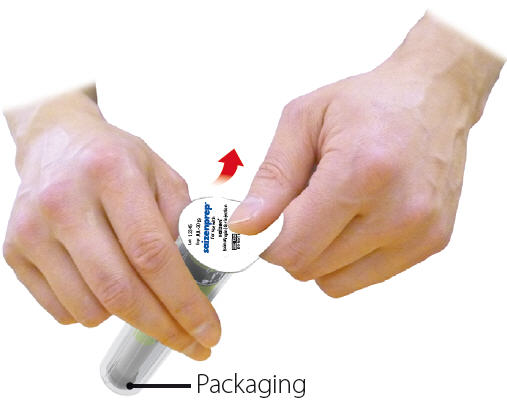
saizenprep® Packaging:
- 2.5
- Peel sterility seal from packaging and discard in the trash.
Caution: Do not attempt to remove device from packaging.
- 2.5
-
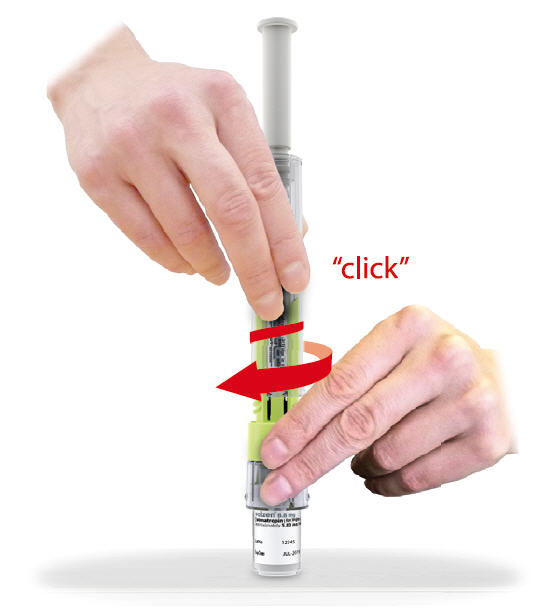
STEP 3
Attach saizenprep® to vial
- 3.1
- Place saizenprep® on top of vial and push down firmly on packaging until it snaps in place. You will feel and hear a "click" confirming that it is fully inserted.
Caution: Make sure that vial is fully inserted inside vial connector.
- 3.2
- Pull off packaging with 1 hand while holding vial with the other hand. Discard packaging in the trash.
- 3.1
-

- 3.2
-

STEP 4
Attach cartridge
- 4.1
- Hold the green cartridge holder with one hand. With the other hand unscrew the transfer unit and set aside for future use.
Note: To enhance cartridge content visibility, line up clear cartridge label space with cartridge holder open slot.
- 4.2
- Insert cartridge into cartridge holder opening with metal tip facing down. You will feel and hear a "click" confirming that it is fully inserted.
- 4.1
-

- 4.2
-

- 4.3
- Screw transfer unit back onto cartridge holder. You will feel and hear a "click" confirming that it is attached correctly.
Caution: Let the plunger rod rise by itself from the transfer unit during attachment step.
- 4.3
-
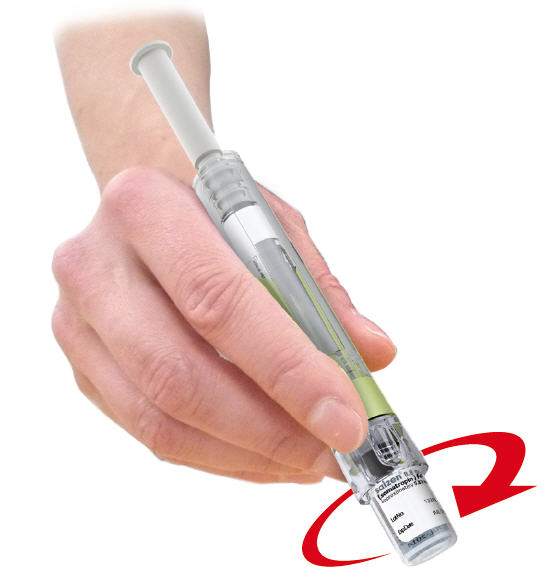
STEP 5
Mix SAIZEN and Diluent in vial
Caution: To avoid foaming, always perform transfers slowly (minimum 10 sec).
- 5.1
- Hold the device at a slight angle. Slowly push down on plunger rod to transfer all Diluent drop by drop to vial. You will feel some resistance. Release plunger rod after transfer is completed. Plunger rod will rise on its own once released.
Warning: You may have to perform this step again if there is still Diluent visible in cartridge.
- 5.1
-

- 5.2
- Thoroughly mix SAIZEN and Diluent in vial by gently swirling device in circular motion.
Do not shake!
Shaking will cause foaming.
Inspect vial:
IF THEN Liquid in vial is clear Proceed to Step 6 Liquid in vial is foaming Allow device to rest vertically until foam disappears (3-5 minutes) Powder in vial is not completely dissolved Continue to swirl gently until drug dissolves (less than 2 minutes) Liquid in vial is cloudy or has particles Do not use and call 1-800-582-7989 - 5.2
-
STEP 6
Draw mixed SAIZEN into cartridge
Caution: Hold saizenprep® straight up - not at an angle when transferring SAIZEN.
- 6.1
- Turn saizenprep® so vial is on top. Push up on plunger rod to remove all air from cartridge. You may feel some resistance.
- 6.2
- Slowly pull plunger rod halfway down. You will start noticing mixed SAIZEN flowing into cartridge.
Note: You may not have to pull as plunger rod may travel back on its own.
- 6.1
-
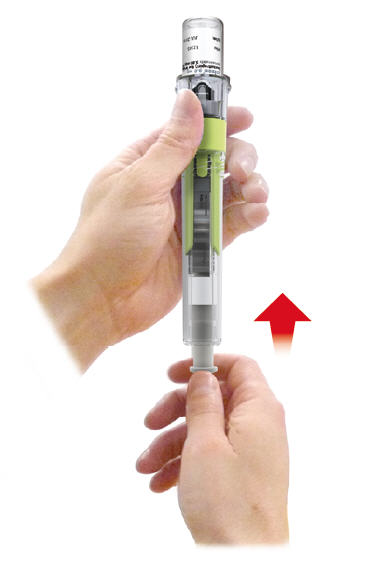
- 6.2
-

- 6.3
- Push up on plunger rod again to remove any remaining air inside cartridge.
- 6.4
- Slowly pull plunger rod downward until it stops. All liquid should now be in the cartridge.
Note: It is ok to leave a small amount of air bubbles in the vial.
- 6.3
-

- 6.4
-

- 6.5
- Hold the device so the top of the cartridge is at eye level and inspect for air bubbles inside the cartridge. The liquid should be at or above the cartridge shoulder line.
If the cartridge contains a large air bubble (see example pg 33) repeat steps 6.3 and 6.4.
Note: If a large air bubble (liquid is below cartridge shoulder line) is not removed after 5 attempts call patient support services at 1-800-582-7989 for assistance. - 6.5
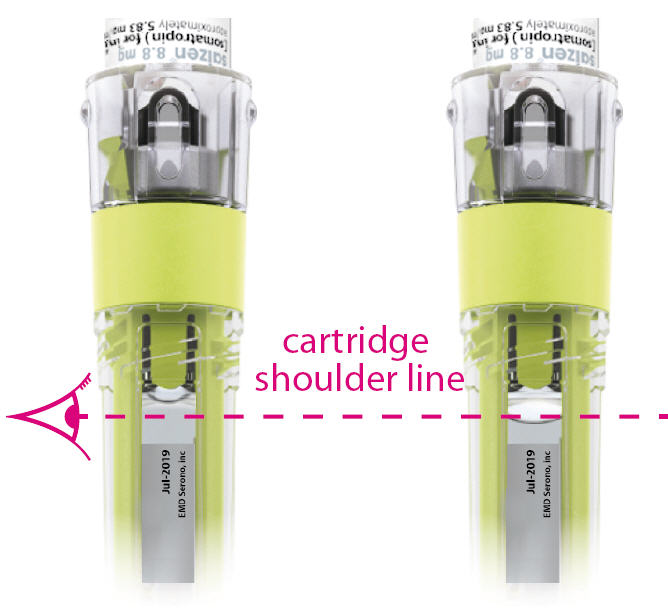
IF
Liquid is at or above cartridge shoulder lineIF
Liquid is below cartridge shoulder lineTHEN
Proceed to step 7THEN
Repeat steps 6.3 and 6.4STEP 7
Remove cartridge
- 7.1
- Turn saizenprep® so vial is on bottom. Hold cartridge holder with one hand and unscrew transfer unit from cartridge holder with other hand. Discard transfer unit in the trash.
Caution: Make sure to hold the cartridge holder when removing the cartridge.
- 7.2
- Remove cartridge from cartridge holder by pulling it straight up.
Note: A small drop of SAIZEN may be visible inside cartridge holder opening.
- 7.1
-

- 7.2
-

- 7.3
- Put the used cartridge holder with attached vial in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the cartridge holder with attached vial in your household trash.
If you do not have an FDA-cleared sharps container, you may use a household container that is: made of a heavy-duty plastic, can be closed with a tight-fitting lid, puncture-resistant lid, without sharps being able to come out, upright and stable during use, leak-resistant, and labeled to warn of hazardous waste inside.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: www.fda.gov/safesharpsdisposal
- 7.3
-

STEP 8
Record date
- 8.1
- Write today's date (mixing date) in white box on cartridge inner label.
- 8.1
-

STEP 9
SAIZEN storage and handling
- 9.1
- Store SAIZEN in the refrigerator between 36°F to 46°F (2°C to 8°C) after mixing.
The cartridge will expire in 21 days after mixing (the date written on the cartridge).
SAIZEN vial, Diluent cartridge and mixed SAIZEN can be stored in the refrigerator near food or other medicine.
If you are traveling, keep SAIZEN away from light and use cool packs to maintain refrigerated temperature between 36°F to 46°F (2°C to 8°C).
Warning: Do not freeze SAIZEN.
- 9.1
-

For more information go to www.saizenus.com or call patient support services at 1-800-582-7989
Manufactured for: EMD Serono, Inc.
Rockland, MA 02370
Made in ItalyThis Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: January 2017EMD Serono, Inc.
is a subsidiary of Merck KGaA,
Darmstadt, Germany20067247 MDT-535 US
MC-2724-2017 - PRINCIPAL DISPLAY PANEL - 5 mg Kit Carton
- PRINCIPAL DISPLAY PANEL - 8.8 mg Kit Carton
-
PRINCIPAL DISPLAY PANEL - 8.8 mg Reconstitution Kit Carton
NDC 44087-0016-1
saizen® 8.8 mg
(somatropin) for injection 5.83 mg/mL
Reconstitution Kit
Approximately 5.83 mg/mL of the drug is delivered
For subcutaneous injection
Exclusively for use with designated Saizen® delivery devicesRx only
- 1
- vial Saizen® 8.8 mg
- 1
- cartridge of diluent
- 1
- saizenprep® Reconstitution Device
EMD
SERONO
-
INGREDIENTS AND APPEARANCE
SAIZEN
somatropin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44087-1005 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44087-1005-2 1 in 1 CARTON 01/17/2017 10/31/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 3 mL Part 2 1 VIAL, GLASS 3.5 mL Part 1 of 2 SAIZEN
somatropin injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROPIN (UNII: NQX9KB6PCL) (SOMATROPIN - UNII:NQX9KB6PCL) SOMATROPIN 5 mg in 3 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 34.2 mg in 3 mL PHOSPHORIC ACID (UNII: E4GA8884NN) 1.16 mg in 3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 10/08/1996 Part 2 of 2 BACTERIOSTATIC WATER
bacteriostatic water injectionProduct Information Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 10/08/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 10/08/1996 10/31/2024 SAIZEN
somatropin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44087-1088 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44087-1088-1 1 in 1 CARTON 08/29/2000 03/31/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 3 mL Part 2 1 VIAL, GLASS 3.5 mL Part 1 of 2 SAIZEN
somatropin injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROPIN (UNII: NQX9KB6PCL) (SOMATROPIN - UNII:NQX9KB6PCL) SOMATROPIN 8.8 mg in 3 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 60.2 mg in 3 mL PHOSPHORIC ACID (UNII: E4GA8884NN) 2.05 mg in 3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 10/08/1996 Part 2 of 2 BACTERIOSTATIC WATER
bacteriostatic water injectionProduct Information Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 10/08/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 08/29/2000 03/31/2024 SAIZENPREP
somatropin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:44087-0016 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44087-0016-1 1 in 1 CARTON 01/17/2017 05/31/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 1.51 mL Part 2 1 CARTRIDGE 1.51 mL Part 1 of 2 SAIZENPREP
somatropin injection, powder, lyophilized, for solutionProduct Information Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOMATROPIN (UNII: NQX9KB6PCL) (SOMATROPIN - UNII:NQX9KB6PCL) SOMATROPIN 8.8 mg in 1.51 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 60.2 mg in 1.51 mL PHOSPHORIC ACID (UNII: E4GA8884NN) 2.05 mg in 1.51 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.51 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 10/08/1996 Part 2 of 2 STERILE WATER
sterile water injectionProduct Information Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) METACRESOL (UNII: GGO4Y809LO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.51 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 10/08/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA019764 01/17/2017 05/31/2023 Labeler - EMD Serono, Inc. (088514898)





