Label: CLINDAMYCIN PHOSPHATE aerosol, foam
- NDC Code(s): 63629-8628-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 45802-660
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Clindamycin Phosphate Foam, 1% safely and effectively. See full prescribing information for Clindamycin Phosphate Foam, 1%.
Clindamycin Phosphate Foam, 1%
For Topical Use
Initial U.S. Approval: 1970INDICATIONS AND USAGE
Clindamycin Phosphate Foam, 1% is a lincosamide product indicated for acne vulgaris in patients 12 years and older. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Foam containing 1% clindamycin as clindamycin phosphate. ( 3)
CONTRAINDICATIONS
Clindamycin Phosphate Foam, 1% is contraindicated in individuals with a history of regional enteritis or ulcerative colitis, or a history of antibiotic-associated colitis, (including pseudomembranous colitis). ( 4)
WARNINGS AND PRECAUTIONS
- Colitis: Clindamycin can cause severe colitis, which may result in death. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of clindamycin. Clindamycin Phosphate Foam, 1% should be discontinued if significant diarrhea occurs. ( 5.1)
ADVERSE REACTIONS
The most common adverse reactions (>1%) are headache and application site reactions including burning, pruritus, and dryness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Colitis
5.2 Irritation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Erythromycin
7.2 Neuromuscular Blocking Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Instructions for Use
17.2 Skin Irritation
17.3 Colitis
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Clindamycin Phosphate Foam, 1% is for topical use only, and not for oral, ophthalmic or intravaginal use.
Apply Clindamycin Phosphate Foam, 1% once daily to affected areas after the skin is washed with mild soap and allowed to fully dry. Use enough to cover the entire affected area.
If there has been no improvement after 6 to 8 weeks or if the condition becomes worse, treatment should be discontinued.
The contents of Clindamycin Phosphate Foam, 1% are flammable; avoid fire, flame and/or smoking during and immediately following application.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Colitis
Systemic absorption of clindamycin has been demonstrated following topical use of this product. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical clindamycin. If significant diarrhea occurs, Clindamycin Phosphate Foam, 1% should be discontinued. [ See Adverse Reactions( 6.2). ]
Severe colitis has occurred following oral or parenteral administration of clindamycin with an onset of up to several weeks following cessation of therapy. Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen severe colitis. Severe colitis may result in death.
Studies indicate a toxin(s) produced by Clostridiais one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Stool cultures for Clostridium difficileand stool assay for C. difficiletoxin may be helpful diagnostically.
5.2 Irritation
Clindamycin Phosphate Foam, 1% can cause irritation. Concomitant topical acne therapy should be used with caution since a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents. If irritation or dermatitis occurs, clindamycin should be discontinued.
Avoid contact of Clindamycin Phosphate Foam, 1% with eyes, mouth, lips, other mucous membranes or areas of broken skin. If contact occurs, rinse thoroughly with water.
Clindamycin Phosphate Foam, 1% should be prescribed with caution in atopic individuals.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 439 subjects with mild to moderate acne vulgaris were treated once daily for 12 weeks with clindamycin phosphate foam, 1%.
The incidence of adverse reactions occurring in >1% of the subjects in clinical trials comparing clindamycin phosphate foam, 1% and its vehicle is presented in Table 1.
Table 1: Adverse Reactions Occurring in ≥ 1% of Subjects
Adverse Reactions
Number (%) of Subjects
Clindamycin Phosphate Foam, 1%
N = 439
Vehicle Foam
N = 154
Headache
12 (3%)
1 (1%)
Application site burning
27 (6%)
14 (9%)
Application site pruritus
5 (1%)
5 (3%)
Application site dryness
4 (1%)
5 (3%)
Application site reaction, not otherwise specified
3 (1%)
4 (3%)
In a contact sensitization study, none of the 203 subjects developed evidence of allergic contact sensitization to clindamycin phosphate foam, 1%.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of clindamycin phosphate foam, 1%: application site pain, application site erythema, diarrhea, urticaria, abdominal pain, hypersensitivity, rash, abdominal discomfort, nausea, seborrhea, application site rash, dizziness, pain of skin, colitis (including pseudomembranous colitis), and hemorrhagic diarrhea. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Abdominal pain and gastrointestinal disturbances, as well as gram-negative folliculitis, have also been reported in association with the use of topical formulations of clindamycin. Orally and parenterally administered clindamycin have been associated with severe colitis, which may end fatally.
-
7 DRUG INTERACTIONS
7.1 Erythromycin
Clindamycin Phosphate Foam, 1% should not be used in combination with topical or oral erythromycin-containing products due to possible antagonism to its clindamycin component. In vitrostudies have shown antagonism between these two antimicrobials. The clinical significance of this in vitroantagonism is not known.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B: There are no adequate and well-controlled studies in pregnant women treated with clindamycin phosphate foam, 1%. Clindamycin Phosphate Foam, 1% should be used during pregnancy only if the potential benefit clearly outweighs the potential risk to the fetus.
Reproduction studies have been performed in rats and mice using subcutaneous and oral doses of clindamycin phosphate, clindamycin hydrochloride and clindamycin palmitate hydrochloride. These studies revealed no evidence of fetal harm.
The highest dose used in the rat and mouse teratogenicity studies was equivalent to a clindamycin phosphate dose of 432 mg/kg. For a rat, this dose is 84 fold higher, and for a mouse 42 fold higher, than the anticipated human dose of clindamycin phosphate from clindamycin phosphate foam, 1% based on a mg/m 2comparison.
8.3 Nursing Mothers
It is not known whether clindamycin is excreted in human milk following use of Clindamycin Phosphate Foam, 1%. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
If used during lactation and Clindamycin Phosphate Foam, 1% is applied to the chest, care should be taken to avoid accidental ingestion by the infant.
-
11 DESCRIPTION
Clindamycin Phosphate Foam, 1% contains clindamycin (1%) as clindamycin phosphate.
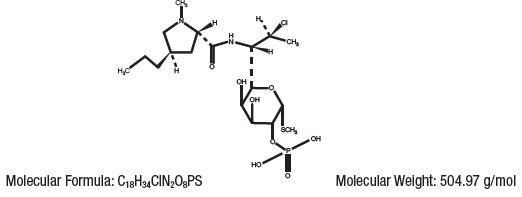
Clindamycin phosphate is a water-soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic, lincomycin.
The chemical name for clindamycin phosphate is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl- trans-4-propyl-L-2-pyrrolidinecarboxamido)1-thio-L- threo-α-D- galacto- octopyranoside 2-(dihydrogen phosphate). The structural formula for clindamycin phosphate is represented below:
Clindamycin Phosphate Foam, 1% contains clindamycin (1%) as clindamycin phosphate, at a concentration equivalent to 10 mg clindamycin per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol, ethanol (58%), polysorbate 60, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mechanism of action in acne vulgaris is unknown. [ See Microbiology (12.4)]
12.3 Pharmacokinetics
In an open label, parallel group study in 24 subjects with acne vulgaris, 12 subjects (3 male and 9 female) applied 4 grams of clindamycin phosphate foam, 1% once-daily for five days, and 12 subjects (7 male and 5 female) applied 4 grams of a clindamycin gel, 1%, once daily for five days. On Day 5, the mean C maxand AUC (0-12)were 23% and 9% lower, respectively, for clindamycin phosphate foam, 1% than for the clindamycin gel, 1%.
Following multiple applications of clindamycin phosphate foam, 1%, less than 0.024% of the total dose was excreted unchanged in the urine over 12 hours on Day 5.
12.4 Microbiology
No microbiology studies were conducted in the clinical trials with this product.
Clindamycin binds to the 50S ribosomal subunits of susceptible bacteria and prevents elongation of peptide chains by interfering with peptidyl transfer, thereby suppressing protein synthesis. Clindamycin has been shown to have in vitroactivity against Propionibacterium acnes( P. acnes), an organism that has been associated with acne vulgaris; however, the clinical significance of this activity against P. acneswas not examined in clinical studies with Clindamycin Phosphate Foam, 1%. P. acnesresistance to clindamycin has been documented.
Inducible Clindamycin Resistance
The treatment of acne with antimicrobials is associated with the development of antimicrobial resistance in P. acnesas well as other bacteria (e.g. Staphylococcus aureus, Streptococcus pyogenes). The use of clindamycin may result in developing inducible resistance in these organisms. This resistance is not detected by routine susceptibility testing.
Cross Resistance
Resistance to clindamycin is often associated with resistance to erythromycin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity of a 1.2% clindamycin phosphate gel similar to Clindamycin Phosphate Foam, 1% was evaluated by daily application to mice for two years. The daily doses used in this study were approximately 3 and 15 times higher than the human dose of clindamycin phosphate from 5 milliliters of Clindamycin Phosphate Foam, 1%, assuming complete absorption and based on a body surface area comparison. No significant increase in tumors was noted in the treated animals.
A 1.2% clindamycin phosphate gel similar to Clindamycin Phosphate Foam, 1% caused a statistically significant shortening of the median time to tumor onset in a study in hairless mice in which tumors were induced by exposure to simulated sunlight.
Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
Reproduction studies in rats using oral doses of clindamycin hydrochloride and clindamycin palmitate hydrochloride have revealed no evidence of impaired fertility.
-
14 CLINICAL STUDIES
In one multicenter, randomized, double-blind, vehicle-controlled clinical trial, subjects with mild to moderate acne vulgaris used clindamycin phosphate foam, 1% or the vehicle Foam once daily for twelve weeks. Treatment response, defined as the proportion of subjects clear or almost clear, based on the Investigator Static Global Assessment (ISGA), and the mean percent reductions in lesion counts at the end of treatment in this study are shown in Table 2.
Table 2: Efficacy Results at Week 12
Efficacy Parameters
Clindamycin Phosphate Foam, 1%
N = 386
Vehicle Foam
N = 127
Treatment response (ISGA)
31%
18%*
Percent reduction in lesion counts
Inflammatory Lesions
49%
35%*
Noninflammatory Lesions
38%
27%*
Total Lesions
43%
31%*
*P<0.05
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
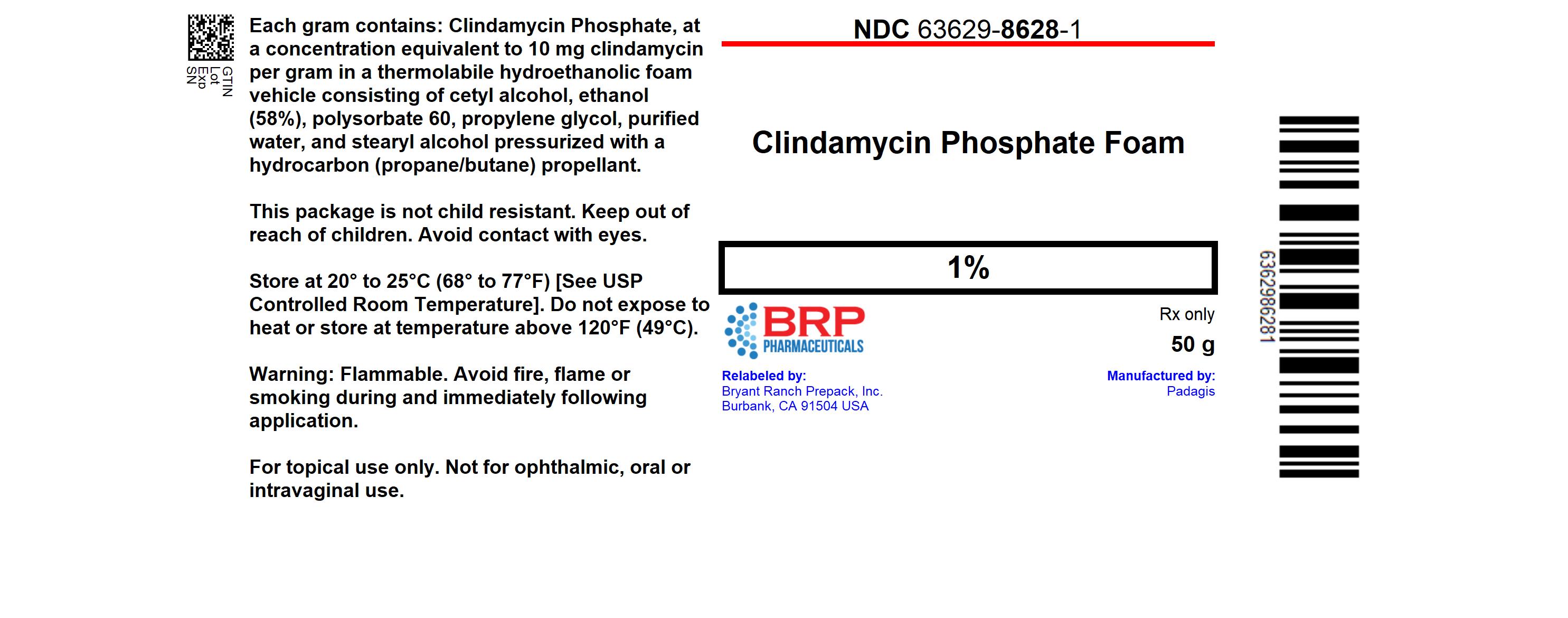
Clindamycin Phosphate Foam, 1% containing clindamycin phosphate equivalent to 10 mg clindamycin per gram, is white to off-white in color and thermolabile.It is available in the following sizes:
- 50 gram aerosol can - NDC 63629-8628-1
16.2 Storage and Handling
Store at 20-25°C (68-77°F) [see USP controlled room temperature].Flammable. Avoid fire, flame or smoking during and immediately following application.
Contents under pressure. Do not puncture or incinerate. Do not expose to heat or store at temperature above 120°F (49°C).
Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved patient labeling (Patient Information).
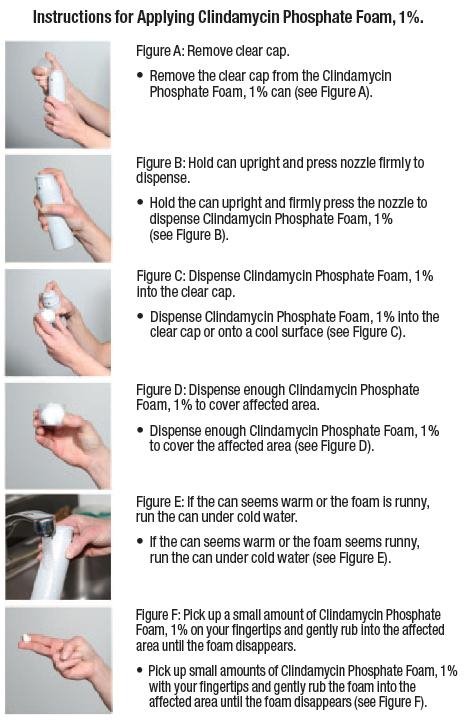
17.1 Instructions for Use
- Patients should be advised to wash their skin with mild soap and allow it to dry before applying Clindamycin Phosphate Foam, 1%.
- Patients should use enough Clindamycin Phosphate Foam, 1% to cover the face and to apply once daily.
- Patients should dispense Clindamycin Phosphate Foam, 1% directly into the cap or onto a cool surface.
Patients should wash their hands after applying Clindamycin Phosphate Foam, 1%.
17.2 Skin Irritation
Clindamycin Phosphate Foam, 1% may cause irritation such as erythema, scaling, itching, burning, or stinging. Patients should be advised to discontinue use if excessive irritancy or dermatitis occur.
17.3 Colitis
In the event a patient treated with Clindamycin Phosphate Foam, 1% experiences severe diarrhea or gastrointestinal discomfort, Clindamycin Phosphate Foam, 1% should be discontinued and a physician should be contacted.
Made in Israel
Manufactured by Perrigo
Yeruham, Israel
Distributed by Perrigo
Allegan, MI 49010
www.perrigo.com
Rev 08-19
4T500 RC J4
Pharmacist-Detach here and Give Instructions to Patient
-
Patient Information
Clindamycin Phosphate Foam, 1%
Important: For skin use only. Do not use Clindamycin Phosphate Foam, 1% in your eyes, mouth or vagina.
Read the Patient Information that comes with Clindamycin Phosphate Foam, 1% before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or treatment.
What is Clindamycin Phosphate Foam, 1%?
Clindamycin Phosphate Foam, 1% is a prescription medicine used on the skin (topical) to treat acne in people 12 years and older.
It is not known if Clindamycin Phosphate Foam, 1% is safe and effective in children under 12 years of age.
Who should not use Clindamycin Phosphate Foam, 1%?
Do not use Clindamycin Phosphate Foam, 1% if you:
- have Crohn’s disease
- have ulcerative colitis
- have had inflammation of the colon (colitis) or severe diarrhea with past antibiotic use
Tell your doctor if you are not sure if you have anyof the conditions listed above.
What should I tell my doctor before using Clindamycin Phosphate Foam, 1%? Before you use Clindamycin Phosphate Foam, 1%, tell your doctor if you:
- have a history of eczema
- are planning to have surgery. Clindamycin Phosphate Foam, 1% may affect how certain medicines work that may be given during surgery.
- have any other medical conditions
- are pregnant or planning to become pregnant. It is not known if Clindamycin Phosphate Foam, 1% may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Clindamycin Phosphate Foam, 1% passes through your breast milk.
You and your doctor should decide if you will use Clindamycin Phosphate Foam, 1% or breastfeed. If you use Clindamycin Phosphate Foam, 1% while breastfeeding and Clindamycin Phosphate Foam, 1% is applied on the chest, take care to avoid getting Clindamycin Phosphate Foam, 1% into your baby’s mouth.
Tell your doctor about all the medicines you takeincluding prescription and non-prescription medicines, vitamins and herbal supplements. Clindamycin Phosphate Foam, 1% may affect the way other medicines work and other medicines may affect how Clindamycin Phosphate Foam, 1% works.
Especially tell your doctor if you take erythromycin or use products on your skin that contain erythromycin.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use Clindamycin Phosphate Foam, 1%?
- Clindamycin Phosphate Foam, 1% is for use on the skin only. Do not get Clindamycin Phosphate Foam, 1% in your eyes, mouth or vagina.
- Use Clindamycin Phosphate Foam, 1% exactly as your doctor tells you to use it. See the “Instructions for Applying Clindamycin Phosphate Foam, 1%” below.
- Apply Clindamycin Phosphate Foam, 1% 1 time a day.
- Wash your skin with mild soap and water and dry before applying Clindamycin Phosphate Foam, 1%.
- Do not dispense Clindamycin Phosphate Foam, 1% directly onto your hands or face, because the foam will begin to melt on contact with warm skin.
- Wash your hands after applying Clindamycin Phosphate Foam, 1%.
Throw away any of the unused medicine that you dispensed out of the can.
What should I avoid while using Clindamycin Phosphate Foam, 1%?
- Clindamycin Phosphate Foam, 1% is flammable. Avoid fire, flames, or smoking during and right after you apply Clindamycin Phosphate Foam, 1% to your skin.
- Avoid getting Clindamycin Phosphate Foam, 1% in or near your eyes, mouth, lips, or broken skin. If you get Clindamycin Phosphate Foam, 1% in your eyes, mouth, on lips or broken skin, rinse well with water.
What are possible side effects with Clindamycin Phosphate Foam, 1%? Clindamycin Phosphate Foam, 1% can cause serious side effects including:
- Inflammation of the colon (colitis). Clindamycin can cause severe colitis that may lead to death. Stop using Clindamycin Phosphate Foam, 1% and call your doctor right away if you have severe watery diarrhea, or bloody diarrhea.
The most common side effects of Clindamycin Phosphate Foam, 1% include:
- Skin irritation. Clindamycin Phosphate Foam, 1% may cause skin irritation such as burning, itching, or dryness. Stop using Clindamycin Phosphate Foam, 1% and talk with your doctor if you develop excessive skin irritation.
- Headache.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the side effects of Clindamycin Phosphate Foam, 1%. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may report side effects to Perrigo at 1-866-634-9120.
How should I store Clindamycin Phosphate Foam, 1%?
- Store Clindamycin Phosphate Foam, 1% at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Clindamycin Phosphate Foam, 1% away from heat. Never throw the can into a fire, even if the can is empty.
- Do not store Clindamycin Phosphate Foam, 1% at temperatures above 120°F (49°C).
- Do not break through (puncture) the Clindamycin Phosphate Foam, 1% can.
Keep Clindamycin Phosphate Foam, 1% and all medicines out of the reach of children.
General information about the safe and effective use of Clindamycin Phosphate Foam, 1%:
Medicines are sometimes prescribed for purposes other than those listed in Patient Information. Do not use Clindamycin Phosphate Foam, 1% for a condition for which it was not prescribed. Do not give Clindamycin Phosphate Foam, 1% to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Clindamycin Phosphate Foam, 1%. If you would like more information, talk with your doctor. You can also ask your pharmacist or doctor for information about Clindamycin Phosphate Foam, 1% that is written for health professionals.
What are the ingredients in Clindamycin Phosphate Foam, 1%?
Active ingredient: clindamycin phosphate
Inactive ingredients: cetyl alcohol, ethanol (58%), polysorbate 60, propylene glycol, purified water, and stearyl alcohol. The can is pressurized with a hydrocarbon (propane/butane) propellant.
This Patient Information has been approved by the U.S. Food and Drug Administration.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLINDAMYCIN PHOSPHATE
clindamycin phosphate aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-8628(NDC:45802-660) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 10 mg in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETYL ALCOHOL (UNII: 936JST6JCN) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-8628-1 1 in 1 CARTON 03/31/2010 1 50 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090785 03/31/2010 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 repack(63629-8628) , relabel(63629-8628)