Label: GAMIFANT- emapalumab-lzsg injection
-
NDC Code(s):
66658-501-01,
66658-505-01,
66658-510-01,
66658-522-01, view more66658-523-01, 66658-524-01, 66658-525-01
- Packager: Swedish Orphan Biovitrum AB (publ)
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GAMIFANT safely and effectively. See full prescribing information for GAMIFANT.
GAMIFANT® (emapalumab-lzsg) injection, for intravenous use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
GAMIFANT is an interferon gamma (IFNγ) blocking antibody indicated for the treatment of adult and pediatric (newborn and older) patients with primary hemophagocytic lymphohistiocytosis (HLH) with refractory, recurrent or progressive disease or intolerance with conventional HLH therapy. (1)
DOSAGE AND ADMINISTRATION
For intravenous infusion only:
DOSAGE FORMS AND STRENGTHS
Injection:
- 10 mg/2 mL (5 mg/mL) solution in a single-dose vial (3)
- 50 mg/10 mL (5 mg/mL) solution in a single-dose vial (3)
- 100 mg/20 mL (5 mg/mL) solution in a single-dose vial (3)
- 50 mg/2 mL (25 mg/mL) solution in a single-dose vial (3)
- 100 mg/4 mL (25 mg/mL) solution in a single-dose vial (3)
- 250 mg/10 mL (25 mg/mL) solution in a single-dose vial (3)
- 500 mg/20 mL (25 mg/mL) solution in a single-dose vial (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Infections: Monitor patients for signs and symptoms and treat promptly. Test for latent tuberculosis. Administer prophylactic treatment against Herpes Zoster, Pneumocystis jirovecii and fungal infections. (5.1)
- Live Vaccines: Do not administer live or live attenuated vaccines to patients receiving GAMIFANT. (5.2)
- Infusion-Related Reactions: Monitor patients for infusion-related reactions. Interrupt infusion for severe infusion reactions and institute appropriate medical management. (5.3)
ADVERSE REACTIONS
The most common adverse reactions (≥ 20%) were: infections, hypertension, infusion-related reactions, and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact 1-866-773-5274 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Monitoring to Assess Safety
2.3 Pre-Medications and Concomitant Medication Information
2.4 Dose Modification Based on Response
2.5 Instructions for Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infections
5.2 Increased Risk of Infection with Use of Live Vaccines
5.3 Infusion-Related Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Effect of GAMIFANT on Cytochrome P450 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The recommended starting dose of GAMIFANT is 1 mg/kg given as an intravenous infusion over 1 hour twice per week (every three to four days). Doses subsequent to the initial dose may be increased based on clinical and laboratory criteria [see Dosage and Administration (2.4)].
Administer GAMIFANT until hematopoietic stem cell transplantation (HSCT) is performed or unacceptable toxicity. Discontinue GAMIFANT when a patient no longer requires therapy for the treatment of HLH.
2.2 Monitoring to Assess Safety
Before Initiating GAMIFANT Treatment
Conduct testing for latent tuberculosis infections using the purified protein derivative (PPD) or IFNγ release assay and evaluate patients for tuberculosis risk factors prior to initiating GAMIFANT. Administer tuberculosis prophylaxis to patients at risk for tuberculosis, or known to have a positive PPD test result, or positive IFNγ release assay.
2.3 Pre-Medications and Concomitant Medication Information
Pre-Medications
Administer prophylaxis for Herpes Zoster, Pneumocystis jirovecii, and for fungal infections prior to GAMIFANT administration.
Concomitant Medications
For patients who are not receiving baseline dexamethasone treatment, begin dexamethasone at a daily dose of at least 5 to 10 mg/m2 the day before GAMIFANT treatment begins. For patients who were receiving baseline dexamethasone, they may continue their regular dose provided the dose is at least 5 mg/m2. Dexamethasone can be tapered according to the judgment of the treating physician [see Clinical Studies (14)].
2.4 Dose Modification Based on Response
The GAMIFANT dose may be titrated up if disease response is unsatisfactory (see Table 1) [see Clinical Pharmacology (12.3)]. After the patient's clinical condition is stabilized, decrease the dose to the previous level to maintain clinical response.
Table 1: Dose Titration Criteria Treatment Day GAMIFANT Dose Criteria for Dose Increase Day 1 Starting Dose of 1 mg/kg N/A On Day 3 Increase to 3 mg/kg Unsatisfactory improvement in clinical condition, as assessed by a healthcare provider AND at least one of the following:
- Fever – persistence or recurrence
- Platelet count
- If baseline < 50,000/mm3 and no improvement to > 50,000/mm3
- If baseline > 50,000/mm3 and less than 30% improvement
- If baseline > 100,000/mm3 and decrease to < 100,000/mm3
- Neutrophil count
- If baseline < 500/mm3 and no improvement to > 500/mm3
- If baseline > 500-1000/mm3 and decrease to < 500/mm3
- If baseline 1000-1500/mm3 and decrease to < 1000/mm3
- Ferritin (ng/mL)
- If baseline ≥ 3000 ng/mL and < 20% decrease
- If baseline < 3000 ng/mL and any increase to > 3000 ng/mL
- Splenomegaly – any worsening
- Coagulopathy (both D-Dimer and Fibrinogen must apply)
- D-Dimer
- If abnormal at baseline and no improvement
- Fibrinogen (mg/dL)
- If baseline levels ≤ 100 mg/dL and no improvement
- If baseline levels > 100 mg/dL and any decrease to < 100 mg/dL
From Day 6 onwards Increase to 6 mg/kg From Day 9 onwards Increase to 10 mg/kg Assessment by a healthcare provider that based on initial signs of response, a further increase in GAMIFANT dose can be of benefit 2.5 Instructions for Preparation and Administration
Preparation
GAMIFANT vials are for single-use only.
Do not mix GAMIFANT 5 mg/mL and 25 mg/mL vials together.
Prepare the solution for infusion as follows:
- Calculate the dose (mg/kg), total volume (mL) of GAMIFANT required and the number of GAMIFANT vials needed based on patient actual body weight [see Dosage and Administration (2.1)].
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. GAMIFANT is a clear to slightly opalescent, colorless to slightly yellow liquid. Do not administer if discolored or foreign particulate matter is present.
- Withdraw the necessary amount of GAMIFANT solution and dilute with 0.9% Sodium Chloride Injection, USP.
- For GAMIFANT 5 mg/mL vials:
- Dilute to a maximum concentration of 2.5 mg/mL. Do not dilute product to less than 0.25 mg/mL.
- The diluted solution can be placed in either a syringe or an infusion bag, depending on the volume needed.
- When using a syringe, use a gamma irradiated latex-free, polyvinyl chloride (PVC)-free syringe. Do not use with ethylene oxide-sterilized syringes.
- When using an infusion bag, use a non-PVC polyolefin infusion bag.
- For GAMIFANT 25 mg/mL vials:
- Dilute to a maximum concentration of 12.5 mg/mL. Do not dilute product to less than 1.25 mg/mL.
- Use a non-PVC polyolefin infusion bag made from either polyethylene or ethylene/propylene copolymer.
- For GAMIFANT 5 mg/mL vials:
- Discard any unused portion left in the vial(s).
Administration
- Administer GAMIFANT diluted solution intravenously over 1 hour through an intravenous line containing a sterile, non-pyrogenic, low-protein binding 0.2 micron in-line filter.
- Do not infuse GAMIFANT concomitantly with other agents and do not add any other product to the infusion bag or syringe.
- Do not store any unused portion of the infusion solution for reuse. Any unused product or waste material should be disposed of in accordance with local requirements.
Storage of Diluted Solution
This product does not contain a preservative.
If not administered immediately:
- Store the diluted solution of GAMIFANT under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 4 hours from the time of dilution.
- If refrigerated, allow the diluted solution to come to room temperature prior to administration.
- Do not freeze. Do not shake.
-
3 DOSAGE FORMS AND STRENGTHS
GAMIFANT is a clear to slightly opalescent, colorless to slightly yellow preservative-free solution available as:
Injection:
- 10 mg/2 mL (5 mg/mL) in a single-dose vial
- 50 mg/10 mL (5 mg/mL) in a single-dose vial
- 100 mg/20 mL (5 mg/mL) in a single-dose vial
- 50 mg/2 mL (25 mg/mL) in a single-dose vial
- 100 mg/4 mL (25 mg/mL) in a single-dose vial
- 250 mg/10 mL (25 mg/mL) in a single-dose vial
- 500 mg/20 mL (25 mg/mL) in a single-dose vial
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Infections
GAMIFANT may increase the risk of fatal and serious infections to include specific pathogens favored by IFNγ neutralization, including mycobacteria, Herpes Zoster virus, and Histoplasma Capsulatum.
Do not administer GAMIFANT in patients with infections caused by these pathogens until appropriate treatment has been initiated.
In 32% of patients receiving GAMIFANT in clinical trials, serious infections such as sepsis, pneumonia, bacteremia, disseminated histoplasmosis, necrotizing fasciitis, viral infections, and perforated appendicitis were observed. The reported infections were viral (41%), bacterial (35%), fungal (9%), and the pathogen was not identified in 15% of cases.
Evaluate patients for tuberculosis risk factors and test for latent infection (PPD testing, PCR, or IFNγ release assay) prior to initiating GAMIFANT. Administer tuberculosis prophylaxis to patients at risk for tuberculosis or known to have a positive purified protein derivative (PPD) test result [see Dosage and Administration (2.2)].
Administer prophylaxis for Herpes Zoster, Pneumocystis jirovecii, and fungal infection to mitigate the risk to patients while receiving GAMIFANT. Employ surveillance testing during treatment with GAMIFANT.
Closely monitor patients receiving GAMIFANT for signs or symptoms of infection, promptly initiate a complete diagnostic workup appropriate for an immunocompromised patient, and initiate appropriate antimicrobial therapy.
5.2 Increased Risk of Infection with Use of Live Vaccines
Do not administer live or live attenuated vaccines to patients receiving GAMIFANT and for at least 4 weeks after the last dose of GAMIFANT. The safety of immunization with live vaccines during or following GAMIFANT therapy has not been studied.
5.3 Infusion-Related Reactions
Infusion-related reactions including drug eruption, pyrexia, rash, erythema, and hyperhidrosis were reported with GAMIFANT treatment in 27% of patients. In one-third of these patients, the infusion-related reaction occurred during the first infusion.
All infusion related reactions were reported as mild to moderate. Monitor patients for infusion- related reactions. Interrupt infusion for infusion reactions and institute appropriate medical management prior to continuing infusion at a slower rate.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Infections [see Warnings and Precautions (5.1)]
- Infusion-Related Reactions [see Warnings and Precautions (5.3)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflect exposure to GAMIFANT in which 34 patients with untreated primary HLH and previously treated patients with primary HLH (NCT01818492) received GAMIFANT at a starting dose of 1 mg/kg every 3 days with dose increases up to 10 mg/kg [see Dosage and Administration (2.1) and Clinical Studies (14)]. The median duration of treatment with GAMIFANT was 59 days (range: 4 to 245 days) and the median cumulative dose was 25 mg/kg (range: 4 to 254 mg/kg).
The median age of study population was 1 year (range: 0.1 to 13 years), 53% were female, and 65% were Caucasian.
Serious adverse reactions were reported in 53% of patients. The most common serious adverse reactions (≥ 3%) included infections, gastrointestinal hemorrhage, and multiple organ dysfunction. Fatal adverse reactions occurred in two (6%) of patients and included septic shock and gastrointestinal hemorrhage.
Disseminated histoplasmosis led to drug discontinuation in one patient. The most commonly reported adverse reactions (≥ 20%) were infections, hypertension, infusion-related reactions, and pyrexia. Adverse reactions reported in ≥ 10% of patients during treatment with GAMIFANT are presented in Table 2.
Table 2: Adverse Reactions Reported in ≥ 10% of Patients with Primary HLH aIncludes viral, bacterial, fungal, and infections in which no pathogen was identified
bIncludes secondary hypertension
cIncludes events of drug eruption, pyrexia, rash, erythema, and hyperhidrosis
Adverse Reactions GAMIFANT
(%)
(N = 34)Infectionsa 56 Hypertensionb 41 Infusion-related reactionsc 27 Pyrexia 24 Hypokalemia 15 Constipation 15 Rash 12 Abdominal pain 12 Cytomegalovirus infection 12 Diarrhea 12 Lymphocytosis 12 Cough 12 Irritability 12 Tachycardia 12 Tachypnea 12 Additional selected adverse reactions (all grades) that were reported in less than 10% of patients treated with GAMIFANT included: vomiting, acute kidney injury, asthenia, bradycardia, dyspnea, gastro-intestinal hemorrhage, epistaxis, and peripheral edema.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other emapalumab products may be misleading.
The immunogenicity of emapalumab-lzsg has been evaluated using an electrochemiluminescence-based immunoassay (ECLIA). A total of 64 subjects were evaluated for anti-therapeutic antibodies (ATAs) to emapalumab-lzsg after treatment with GAMIFANT. ATAs were detected in 3/64 subjects (5%) who received GAMIFANT.
Treatment-emergent ATAs were detected in 1/33 (3%) of patients in the primary HLH clinical trial. The ATAs in this patient were found to have neutralizing ability. One patient receiving GAMIFANT through compassionate use developed transient non-neutralizing treatment- emergent ATAs. In both of these patients, ATAs occurred within the first 9 weeks following the initiation of GAMIFANT treatment. In addition, one healthy subject tested positive for ATAs following a single dose of GAMIFANT. No evidence of an altered safety or efficacy profile was identified in the primary HLH patients who developed antibodies to emapalumab-lzsg.
-
7 DRUG INTERACTIONS
7.1 Effect of GAMIFANT on Cytochrome P450 Substrates
The formation of CYP450 enzymes may be suppressed by increased levels of cytokines (such as IFNγ) during chronic inflammation. By neutralizing IFNγ, use of GAMIFANT may normalize CYP450 activities which may reduce the efficacy of drugs that are CYP450 substrates due to increased metabolism.
Upon initiation or discontinuation of concomitant GAMIFANT, monitor for reduced efficacy and adjust dosage of CYP450 substrate drugs as appropriate.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on GAMIFANT use in pregnant women to inform a drug-associated risk of adverse developmental outcomes. In an animal reproduction study, a murine surrogate anti-mouse IFNγ antibody administered to pregnant mice throughout gestation crossed the placental barrier, and no fetal harm was observed (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In a mouse embryo-fetal development study, a murine surrogate anti-mouse IFNγ antibody was administered every 3-4 days throughout organogenesis and late gestation at doses of 0, 30, 75 or 150 mg/kg/occasion. The surrogate antibody was detected in the plasma of all treated pregnant mice and their corresponding fetuses. No maternal toxicity occurred and there was no evidence of teratogenicity or effects on embryo-fetal survival or growth.
8.2 Lactation
Risk Summary
There is no information regarding the presence of emapalumab-lzsg in human milk, the effects on the breastfed child, or the effects on milk production. Published data suggest that only limited amounts of therapeutic antibodies are found in breast milk and they do not enter the neonatal and infant circulations in substantial amounts.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for GAMIFANT and any potential adverse effects on the breastfed child from GAMIFANT or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of GAMIFANT have been established in pediatric patients, newborn and older, with primary HLH that is reactivated or refractory to conventional therapies. Use of GAMIFANT is supported by a single-arm trial in 27 pediatric patients with reactivated or refractory primary HLH. This study included pediatric patients in the following age groups: 5 patients newborn to 6 months, 10 patients 6 months to 2 years, and 12 patients from 2 years to 13 years [see Clinical Studies (14)].
8.5 Geriatric Use
Clinical studies of GAMIFANT did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
11 DESCRIPTION
Emapalumab-lzsg is an interferon gamma (IFNγ) blocking antibody. Emapalumab-lzsg is produced in Chinese Hamster Ovary cells by recombinant DNA technology. Emapalumab-lzsg is an IgG1 immunoglobulin with a molecular weight of approximately 148 kDa.
GAMIFANT (emapalumab-lzsg) injection for intravenous use is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution provided in single-dose vials that require dilution prior to intravenous infusion.
GAMIFANT 5 mg/mL (2 mL, 10 mL and 20 mL)
Each vial contains 10 mg/2 mL, 50 mg/10 mL, or 100 mg/20 mL emapalumab-lzsg at a concentration of 5 mg/mL. Each mL also contains the following inactive ingredients: L-Histidine (1.55 mg), L-Histidine monohydrochloride, monohydrate (3.14 mg), Polysorbate 80 (0.05 mg), sodium chloride (7.30 mg), and Water for Injection, USP.
GAMIFANT 25 mg/mL (2 mL, 4 mL, 10 mL and 20 mL)
Each vial contains 50 mg/2 mL, 100 mg/4 mL, 250 mg/10 mL or 500 mg/20 mL emapalumab- lzsg at a concentration of 25 mg/mL. Each mL also contains the following inactive ingredients: L-Histidine (1.55 mg), L-Histidine monohydrochloride, monohydrate (3.14 mg), Polysorbate 80 (0.05 mg), sodium chloride (7.30 mg), and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Emapalumab-lzsg is a monoclonal antibody that binds to and neutralizes interferon gamma (IFNγ). Nonclinical data suggest that IFNγ plays a pivotal role in the pathogenesis of HLH by being hypersecreted.
12.2 Pharmacodynamics
12.3 Pharmacokinetics
The pharmacokinetics of emapalumab-lzsg were evaluated in healthy adult subjects and in patients with primary HLH.
Following a 1 mg/kg emapalumab-lzsg dose, median steady state peak concentration was 44 mcg/mL, which was 2.9 times higher than after the first dose. The median steady state trough concentration was 25 mcg/mL, which was 4.3 times higher than after the first dose.
Emapalumab-lzsg AUC increases slightly more than proportionally between 1 and 3 mg/kg doses, and less than proportionally at 3, 6, and 10 mg/kg doses.
Emapalumab-lzsg exhibits target-mediated clearance dependent on IFNγ production, which can vary between and within patients as a function of time and can affect the recommended dosage [see Dosage and Administration (2.2)]. Emapalumab-lzsg steady state is achieved by the 7th infusion when the IFNγ production is moderate. At high IFNγ production, steady-state is reached earlier due to a shorter half-life.
Distribution
The central and peripheral volumes of distribution in a subject with body weight of 70 kg are 4.2 and 5.6 L, respectively.
Elimination
Emapalumab-lzsg elimination half-life is approximately 22 days in healthy subjects, and ranged from 2.5 to 18.9 days in HLH patients.
Emapalumab-lzsg clearance is approximately 0.007 L/h in healthy subjects.
In patients, the total clearance of emapalumab-lzsg was significantly influenced by the production of IFNγ, demonstrating target mediated clearance of emapalumab-lzsg.
Metabolism
The metabolic pathway of emapalumab-lzsg has not been characterized. Like other protein therapeutics, GAMIFANT is expected to be degraded into small peptides and amino acids via catabolic pathways.
Specific Populations
Body weight (2 to 82 kg) was a significant covariate of emapalumab-lzsg pharmacokinetics, supporting body weight-based dosing.
No clinically significant differences in the pharmacokinetics of emapalumab-lzsg were observed based on age (0.02 to 56 year), sex (53% Females), race (71.4% Caucasian, 12.2% Asian and 8.2% Black), renal impairment including dialysis, or hepatic impairment (mild, moderate, and severe).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with emapalumab-lzsg.
No studies have been conducted to evaluate the effects of emapalumab-lzsg on fertility; however, no adverse effects on male or female reproductive organs were observed in the 8- or 13-week repeat-dose toxicity studies in cynomolgus monkeys.
-
14 CLINICAL STUDIES
The efficacy of GAMIFANT was evaluated in a multicenter, open-label, single-arm trial NI-0501-04 (NCT01818492) in 27 pediatric patients with suspected or confirmed primary HLH with either refractory, recurrent, or progressive disease during conventional HLH therapy or who were intolerant of conventional HLH therapy.
Patients were required to fulfill the following criteria for enrollment: primary HLH based on a molecular diagnosis or family history consistent with primary HLH or five out of the 8 criteria fulfilled: fever, splenomegaly, cytopenias affecting 2 of 3 lineages in the peripheral blood (hemoglobin < 9 , platelets < 100 x 109/L, neutrophils < 1 x 109/L), hypertriglyceridemia (fasting triglycerides > 3 mmol/L or ≥ 265 mg/dL) and/or hypofibrinogenemia (≤ 1.5 g/L), hemophagocytosis in bone marrow, spleen, or lymph nodes with no evidence of malignancy, low or absent NK-cell activity, ferritin ≥ 500 mcg/L, soluble CD25 ≥ 2400 U/mL. Patients had to have evidence of active disease as assessed by treating physician. Patients had to fulfill one of the following criteria as assessed by the treating physician: having not responded or not achieved a satisfactory response or not maintained a satisfactory response to conventional HLH therapy, or intolerance to conventional HLH treatments. Patients with active infections caused by specific pathogens favored by IFNγ neutralization were excluded from the trial (e.g., mycobacteria and Histoplasma Capsulatum). Patients received prophylaxis for Herpes Zoster, Pneumocystis jirovecii, and fungal infections.
Twenty-seven patients enrolled and received treatment in the study and twenty patients (74%) completed the study. Seven patients (26%) were prematurely withdrawn. Twenty-two patients (81%) enrolled onto the open-label extension study which monitored patients for up to 1 year after HSCT or after the last GAMIFANT infusion (NI-0501-05; NCT02069899).
The study treatment duration was up to 8 weeks after which patients could continue treatment on the extension study. All patients received an initial starting dose of GAMIFANT of 1 mg/kg every 3 days. Subsequent doses could be increased to a maximum of 10 mg/kg based on clinical and laboratory parameters interpreted as unsatisfactory response. Forty-four percent of patients remained at a dose of 1 mg/kg, 30% of patients increased to 3-4 mg/kg and 26% of patients increased to 6-10 mg/kg. The median time to dose increase was 27 days (range: 3-31 days) with 22% of patients requiring a dose increase in the first week of treatment.
All patients received dexamethasone as background HLH treatment with doses between 5 to 10 mg/m2/day. Cyclosporine A was continued if administered prior to screening. Patients receiving methotrexate and glucocorticoids administered intrathecally at baseline could continue these treatments.
In Study NI-0501-04, the median patient age was 1 year (0.2 to 13). Fifty-nine percent of the patients were female, 63% were Caucasian, 11% were Asian, and 11% were Black.
A genetic mutation known to cause HLH was present in 82% of patients. The most frequent causative mutations were FHL3-UNC13D (MUNC 13-4) (26%), FHL2-PRF1 (19%), and Griscelli Syndrome type 2 (19%).
The HLH mutations in the population enrolled are described in Table 3.
Table 3: HLH Mutations in Patients with Primary HLH with Prior Therapy GAMIFANT
(N=27)HLH Genetic Confirmation 22 (82) FHL3 – UNC13D 7 (26) FHL2 – PRF1 5 (19) Griscelli Syndrome type 2 (RAB27A) 5 (19) FHL5 – STXBP2 (UNC18B) 2 (7.4) FHL4 – STX11 1 (3.7) X-linked Lymphoproliferative Disorder
11 (3.7) X-linked Lymphoproliferative Disorder
21 (3.7) All patients received previous HLH treatments. Patients received a median of 3 prior agents before enrollment into the trial. Prior regimens included combinations of the following agents: dexamethasone, etoposide, cyclosporine A, and anti-thymocyte globulin.
At baseline entry into the study, 78% of patients had elevated ferritin levels, thrombocytopenia (70% with platelet count of < 100 x 109cells/L), hypertriglyceridemia (67%) with triglyceride level > 3 mmol/L. Central nervous system findings were present in 37% of patients. Forty-one percent of patients had active infections not due to specific pathogens favored by IFNγ neutralization at the time of GAMIFANT initiation.
The efficacy of GAMIFANT was based upon overall response rate (ORR) at the end of treatment, defined as achievement of either a complete or partial response or HLH improvement. ORR was evaluated using an algorithm that included the following objective clinical and laboratory parameters: fever, splenomegaly, central nervous system symptoms, complete blood count, fibrinogen and/or D-dimer, ferritin, and soluble CD25 (also referred to as soluble interleukin-2 receptor) levels. Complete response was defined as normalization of all HLH abnormalities (i.e., no fever, no splenomegaly, neutrophils > 1x109/L, platelets > 100x109/L, ferritin < 2,000 μg/L, fibrinogen > 1.50 g/L, D-dimer < 500 μg/L, normal CNS symptoms, no worsening of sCD25 > 2-fold baseline). Partial response was defined as normalization of ≥ 3 HLH abnormalities. HLH improvement was defined as ≥ 3 HLH abnormalities improved by at least 50% from baseline.
Table 4: Overall Response Rate at End of Treatment †p-value based on Exact Binomial Test at a one-sided significance level of 2.5% comparing proportion of patients with overall response to hypothesized null hypothesis of 40%.
CI = confidence interval
GAMIFANT
(N=27)Overall Response Rate N (%) 17 (63) (95% CI) (0.42, 0.81) p-value† 0.013 Overall Response by Category Complete response, n (%) 7 (26) Partial response 8 (30) HLH improvement 2 (7.4) The median duration of first response, defined as time from achievement of first response to loss of first response, is not reached (range: 4-56+ days). Seventy percent (19/27) of patients proceeded to HSCT.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GAMIFANT (emapalumab-lzsg) injection is a sterile, clear to slightly opalescent, colorless to slightly yellow solution supplied in the following packaging configuration:
NDC 66658-501-01 – containing one 10 mg/2 mL (5 mg/mL) single-dose vial
NDC 66658-505-01 – containing one 50 mg/10 mL (5 mg/mL) single-dose vial
NDC 66658-510-01 – containing one 100 mg/20 mL (5 mg/mL) single-dose vial
NDC 66658-522-01 – containing one 50 mg/2 mL (25 mg/mL) single-dose vial
NDC 66658-523-01 – containing one 100 mg/4 mL (25 mg/mL) single-dose vial
NDC 66658-524-01 – containing one 250 mg/10 mL (25 mg/mL) single-dose vial
NDC 66658-525-01 – containing one 500 mg/20 mL (25 mg/mL) single-dose vial
Store GAMIFANT in a refrigerator at 2ºC to 8ºC (36ºF to 46ºF) in original carton to protect from light. Do not freeze or shake. This product contains no preservative.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Infections
Inform patients and their caregivers of the risk of developing infections during treatment with GAMIFANT, and to report any symptoms of infection [see Warnings and Precautions (5.1)].
Vaccinations
Advise patients and their caregivers that the patient should not receive live or live attenuated vaccines during GAMIFANT treatment [see Warnings and Precautions (5.2)].
Infusion-Related Reactions
Advise patients and their caregivers of the potential for developing infusion-related reactions during treatment with GAMIFANT [see Warnings and Precautions (5.3)].
Manufactured by:
Swedish Orphan Biovitrum AB (publ)
Stockholm, Sweden
U.S. License Number 1859Distributed by:
Sobi Inc.
77 Fourth Avenue, 3rd Floor
Waltham, MA 02451-7559Manufactured at:
Patheon Italia S.p.A
2° Trav. SX Via Morolense, 5
03013-Ferentino ItalyProduct of the United Kingdom
-
MEDICATION GUIDE
MEDICATION GUIDE
GAMIFANT® (gam' i fant)
(emapalumab-lzsg)
injection, for intravenous useWhat is the most important information I should know about GAMIFANT?
GAMIFANT can cause serious side effects including:
Infections. GAMIFANT is a medicine that affects your immune system and may lower the ability of your immune system to fight infections. GAMIFANT may increase your risk of serious infections that can lead to death. These infections include tuberculosis (TB), histoplasmosis, Herpes zoster infection (shingles) and other infections caused by viruses, fungi or bacteria that can spread throughout the body.
Your healthcare provider will:- test you for TB before you start treatment with GAMIFANT.
- treat you with a medicine for TB if you at risk for TB or if you have a known positive TB test.
Before starting GAMIFANT, tell your healthcare provider if you:- had TB in the past, or if you or a member of your family have been in recent close contact with someone with TB.
- have ever had a positive TB skin test (PPD).
- currently have or have had history of infections, including histoplasmosis or Herpes zoster (shingles).
- are being treated for an active infection.
- have symptoms of an infection, such as fever, sweat and chills, cough, breathing problems, blood in mucus (phlegm), warm, red, or painful skin or sores on your body.
- new symptoms of an infection appear.
- symptoms of an infection that you already had when starting GAMIFANT worsen.
See "What are the possible side effects of GAMIFANT?" for more information about side effects.What is GAMIFANT?
GAMIFANT is a prescription medicine used for the treatment of adults and children (newborn and older) with primary hemophagocytic lymphohistiocytosis (HLH) whose disease has come back or progressed, or other medicines have not worked well enough or cannot be tolerated.Before you receive GAMIFANT, tell your healthcare provider about all of your medical conditions including if you: - have an infection (see "What is the most important information I should know about GAMIFANT?")
- have received or are scheduled to receive an immunization (vaccine). You should not receive "live or attenuated-live" vaccines during your treatment with GAMIFANT and for at least 4 weeks after the last dose of GAMIFANT.
- are pregnant or plan to become pregnant. It is not known if GAMIFANT can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if GAMIFANT passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with GAMIFANT.
How will I receive GAMIFANT? - You will receive GAMIFANT through a vein by intravenous (IV) infusion over 1 hour.
- Your healthcare provider will monitor you during the infusion for side effects.
- GAMIFANT is given 2 times a week (every 3 to 4 days).
- Your healthcare provider will do blood tests during your treatment with GAMIFANT to see how well you respond to treatment.
- GAMIFANT is used with another prescription medicine called dexamethasone. You can ask your healthcare provider for information about dexamethasone.
What are the possible side effects of GAMIFANT?
GAMIFANT can cause serious side effects, including:- See "What is the most important information I should know about GAMIFANT?"
-
Infusion reactions. Infusion reactions are common with GAMIFANT, and can also be severe. Infusion reactions can happen during or shortly after treatment with GAMIFANT. Your healthcare provider may temporarily stop your infusion and treat your symptoms before continuing your infusion if you have severe infusion reactions. Tell your healthcare provider right away if you get any of the following symptoms:
- skin redness
- itching
- fever
- rash
- excessive sweating
- chills
- chest pain
- shortness of breath
- nausea or vomiting
- lightheadedness or dizziness
These are not all of the possible side effects of GAMIFANT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of GAMIFANT.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider or pharmacist for information about GAMIFANT that is written for health professionals.What are the ingredients in GAMIFANT?
Active ingredient: emapalumab-lzsg
Inactive ingredients: L-Histidine, L-Histidine monohydrochloride, monohydrate, Polysorbate 80, sodium chloride, andWater for Injection, USP
Manufactured at: Patheon Italia S.p.A. 2° Trav. SX Via Morolense, 5, 03013-Ferentino Italy
Manufactured by: Swedish Orphan Biovitrum AB (publ), Stockholm, Sweden, U.S. License Number 1859
Distributed by: Sobi Inc., 77 Fourth Avenue, 3rd Floor, Waltham, MA 02451-7559
Product of the United Kingdom
For more information call 1-866-773-5274 or go to www.gamifant.comThis Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 05/2022 -
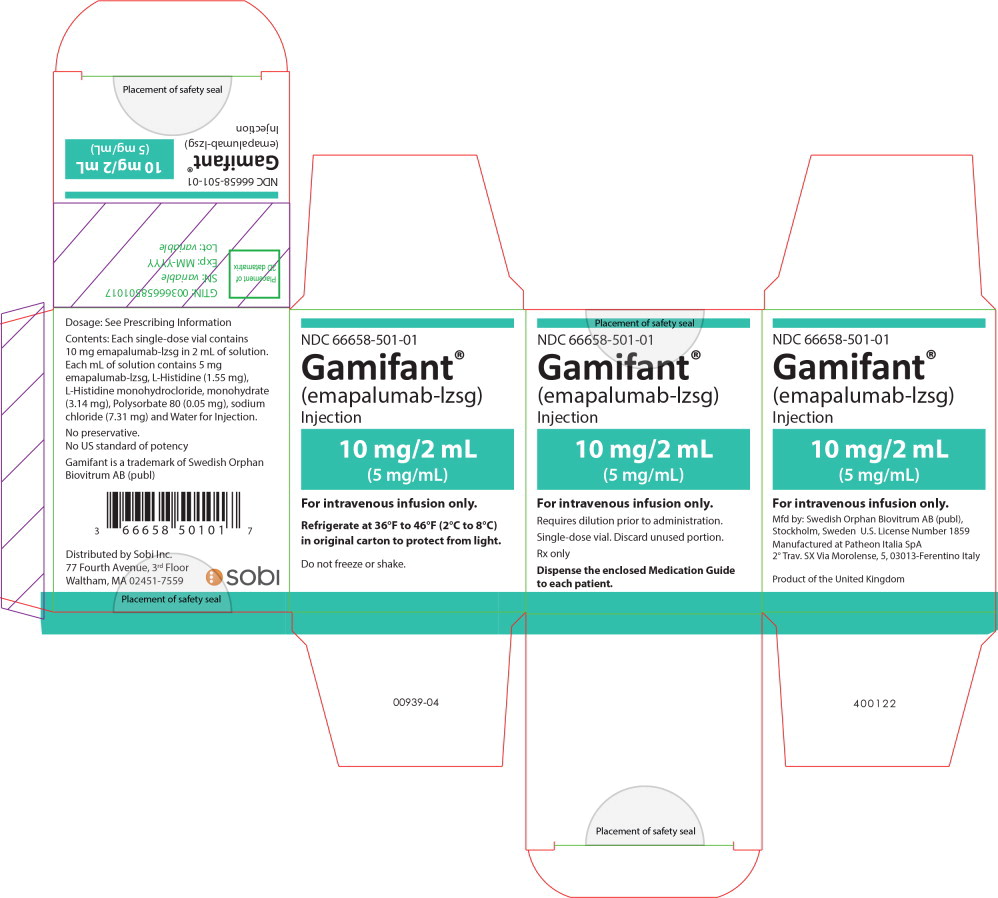
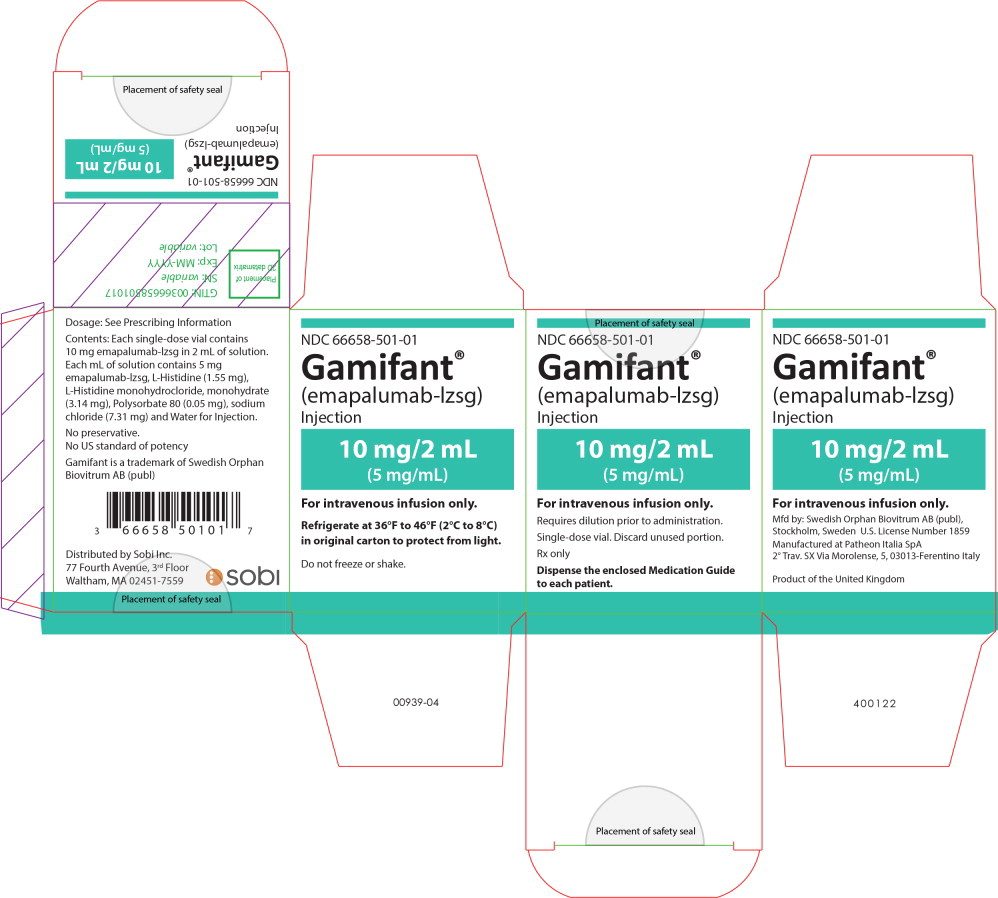
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 10mg/2mL Carton Label
NDC 66658-501-01
Gamifant®
(emapalumab-lzsg)
Injection10 mg/2 mL
(5 mg/mL)For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 50mg/10mL Carton Label
NDC 66658-505-01
Gamifant®
(emapalumab-lzsg)
Injection50 mg/10 mL
(5 mg/mL)For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 100mg/20mL Carton Label
NDC 66658-510-01
Gamifant®
(emapalumab-lzsg)
Injection100 mg/20 mL
(5 mg/mL)For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient. -
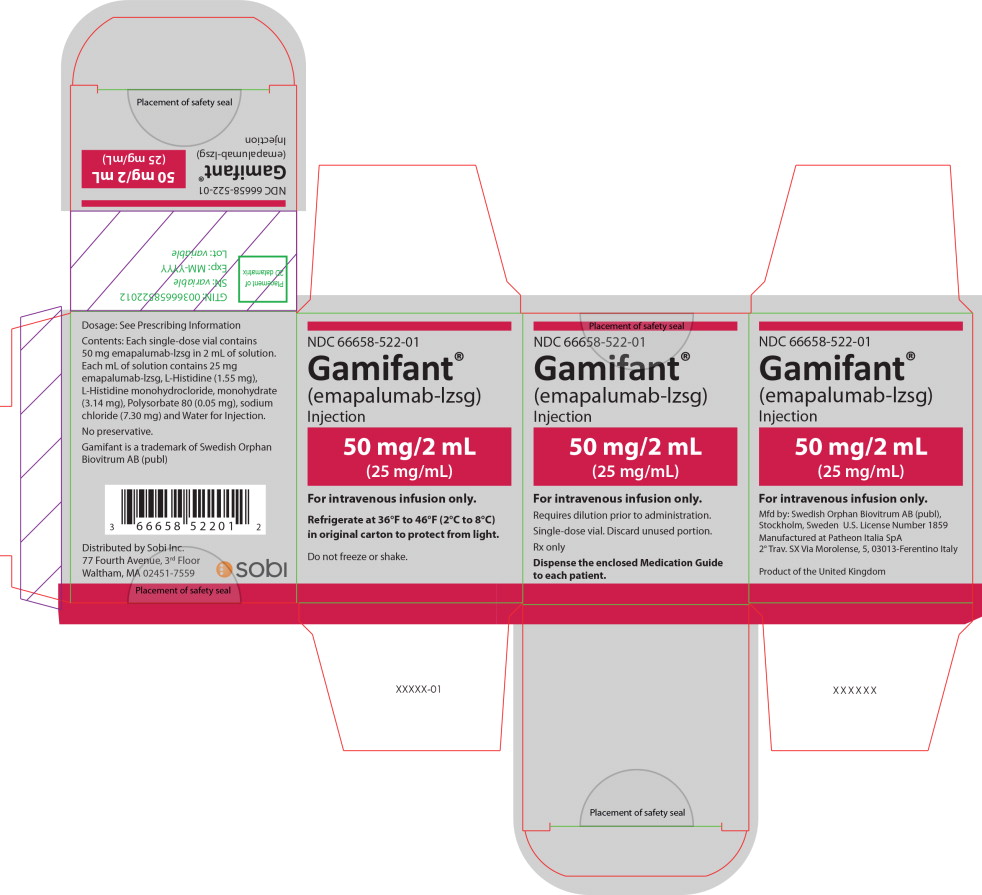
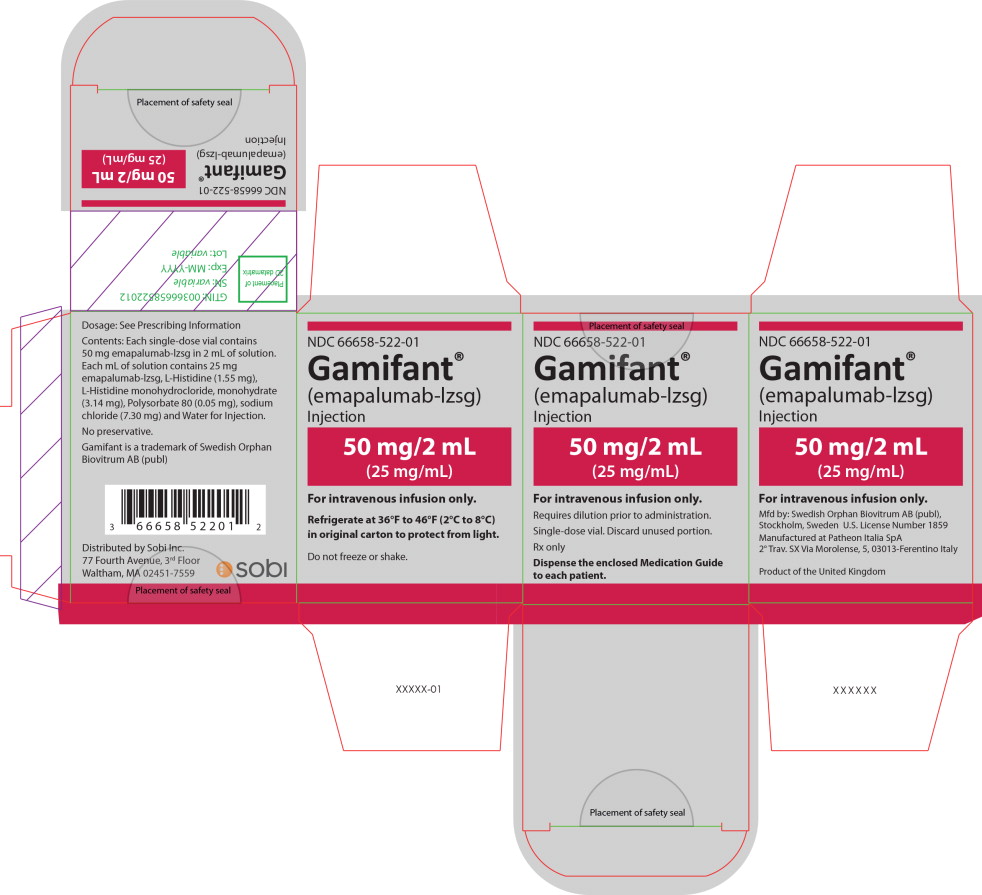
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 50 mg/2 mL Carton Label
NDC 66658-522-01
Gamifant®
(emapalumab-lzsg)
Injection50 mg/2 mL
(25 mg/mL)For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient. -
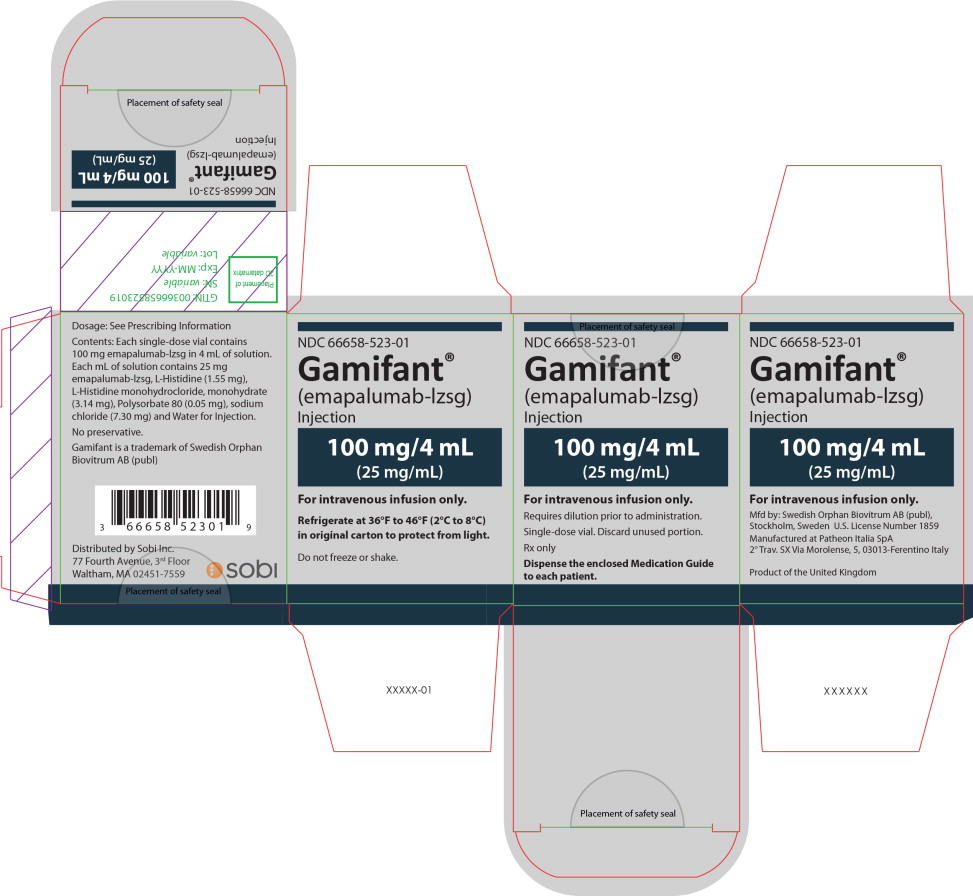
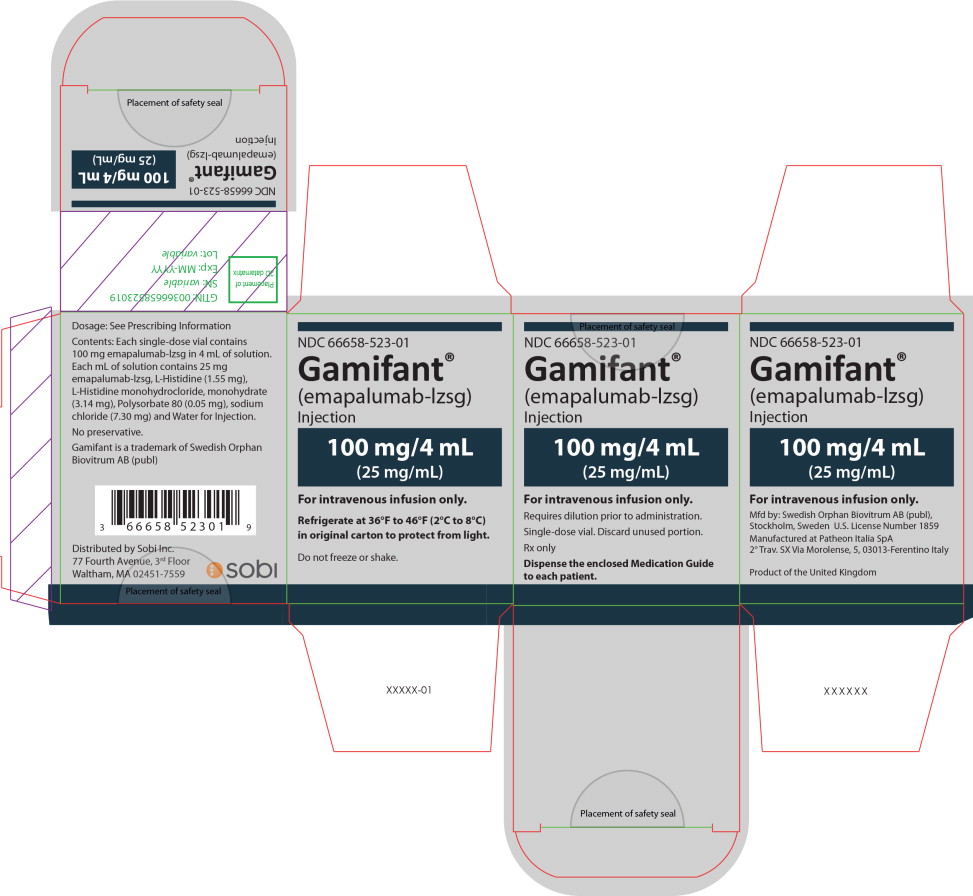
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 100 mg/4 mL Carton Label
NDC 66658-523-01
Gamifant®
(emapalumab-lzsg)
Injection100 mg/4 mL
(25 mg/mL)For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 250 mg/10 mL Carton Label
NDC 66658-524-01
Gamifant®
(emapalumab-lzsg)
Injection250 mg/10 mL
(25 mg/mL)For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient. -
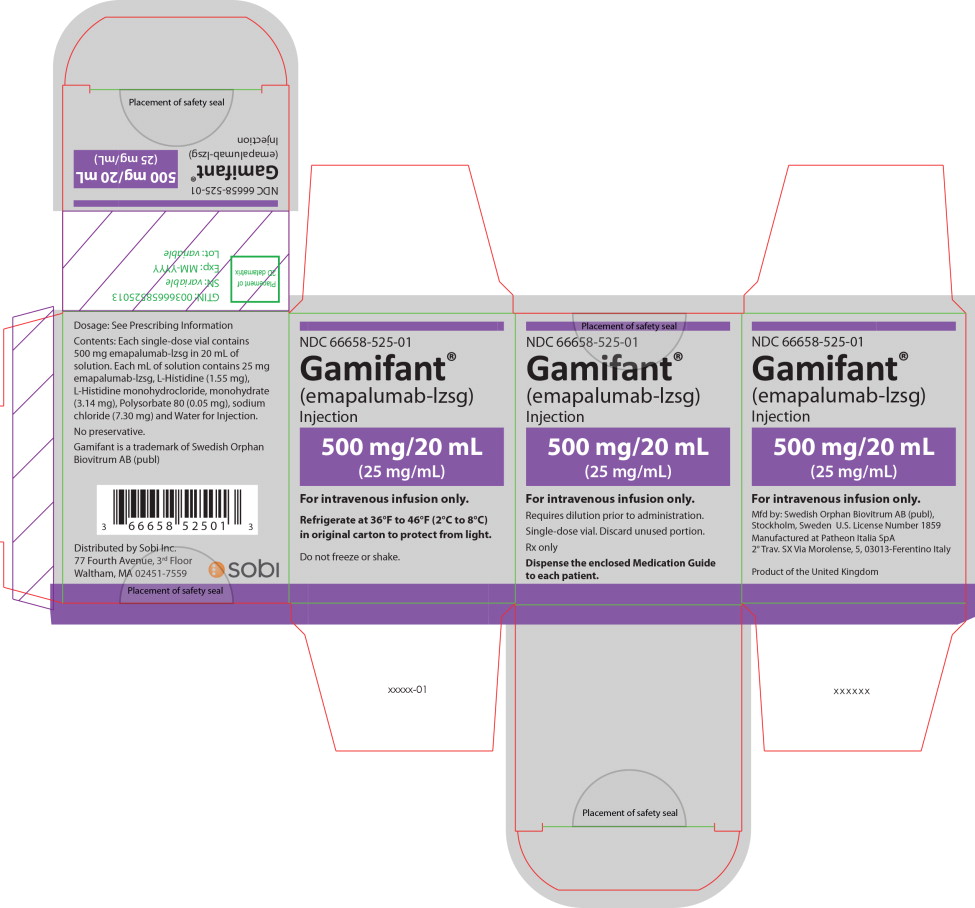
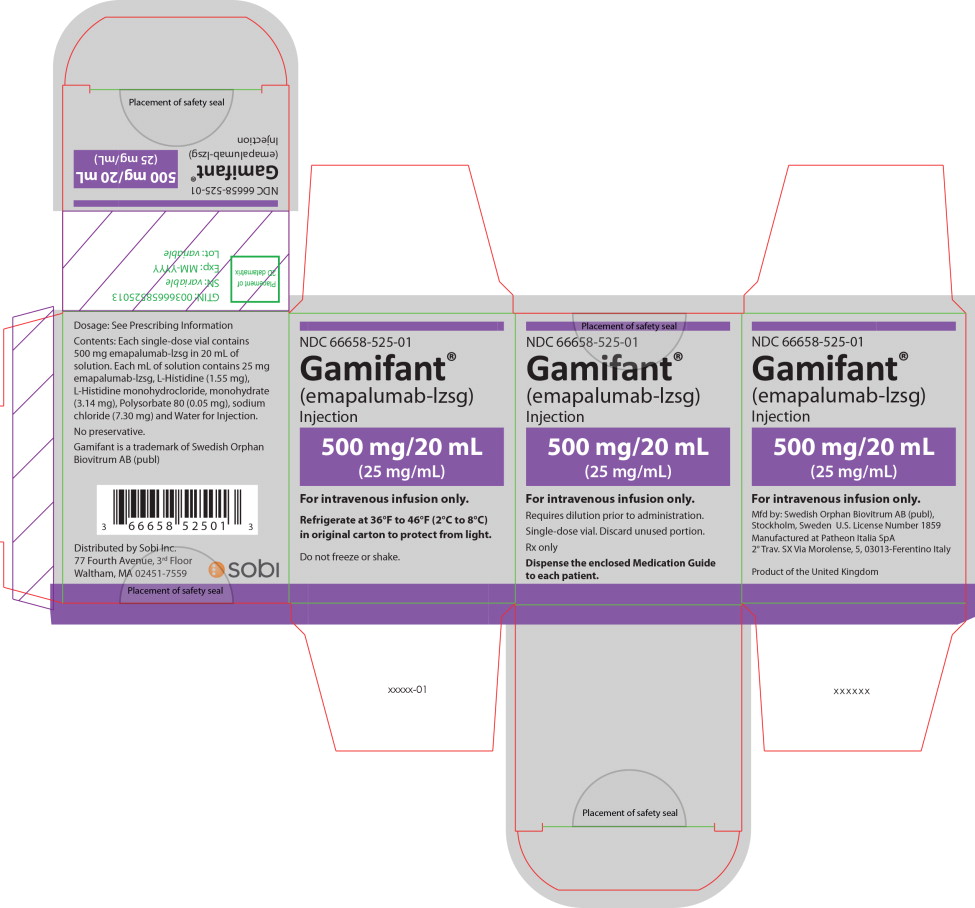
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 500 mg/20 mL Carton Label
NDC 66658-525-01
Gamifant®
(emapalumab-lzsg)
Injection500 mg/20 mL
(25 mg/mL)For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient. -
INGREDIENTS AND APPEARANCE
GAMIFANT
emapalumab-lzsg injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66658-501 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emapalumab (UNII: 3S252O2Z4X) (emapalumab - UNII:3S252O2Z4X) emapalumab 10 mg in 2 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) histidine monohydrochloride monohydrate (UNII: X573657P6P) polysorbate 80 (UNII: 6OZP39ZG8H) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66658-501-01 1 in 1 CARTON 05/17/2019 1 2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761107 05/17/2019 GAMIFANT
emapalumab-lzsg injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66658-505 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emapalumab (UNII: 3S252O2Z4X) (emapalumab - UNII:3S252O2Z4X) emapalumab 50 mg in 10 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) histidine monohydrochloride monohydrate (UNII: X573657P6P) polysorbate 80 (UNII: 6OZP39ZG8H) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66658-505-01 1 in 1 CARTON 05/17/2019 1 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761107 05/17/2019 GAMIFANT
emapalumab-lzsg injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66658-510 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emapalumab (UNII: 3S252O2Z4X) (emapalumab - UNII:3S252O2Z4X) emapalumab 100 mg in 20 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) histidine monohydrochloride monohydrate (UNII: X573657P6P) polysorbate 80 (UNII: 6OZP39ZG8H) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66658-510-01 1 in 1 CARTON 06/26/2020 1 20 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761107 06/26/2020 GAMIFANT
emapalumab-lzsg injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66658-522 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emapalumab (UNII: 3S252O2Z4X) (emapalumab - UNII:3S252O2Z4X) emapalumab 50 mg in 2 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) histidine monohydrochloride monohydrate (UNII: X573657P6P) polysorbate 80 (UNII: 6OZP39ZG8H) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66658-522-01 1 in 1 CARTON 06/07/2023 1 2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761107 06/07/2023 GAMIFANT
emapalumab-lzsg injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66658-523 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emapalumab (UNII: 3S252O2Z4X) (emapalumab - UNII:3S252O2Z4X) emapalumab 100 mg in 4 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) histidine monohydrochloride monohydrate (UNII: X573657P6P) polysorbate 80 (UNII: 6OZP39ZG8H) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66658-523-01 1 in 1 CARTON 06/07/2023 1 4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761107 06/07/2023 GAMIFANT
emapalumab-lzsg injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66658-524 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emapalumab (UNII: 3S252O2Z4X) (emapalumab - UNII:3S252O2Z4X) emapalumab 250 mg in 10 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) histidine monohydrochloride monohydrate (UNII: X573657P6P) polysorbate 80 (UNII: 6OZP39ZG8H) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66658-524-01 1 in 1 CARTON 06/07/2023 1 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761107 06/07/2023 GAMIFANT
emapalumab-lzsg injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66658-525 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength emapalumab (UNII: 3S252O2Z4X) (emapalumab - UNII:3S252O2Z4X) emapalumab 500 mg in 20 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) histidine monohydrochloride monohydrate (UNII: X573657P6P) polysorbate 80 (UNII: 6OZP39ZG8H) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66658-525-01 1 in 1 CARTON 06/07/2023 1 20 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761107 06/07/2023 Labeler - Swedish Orphan Biovitrum AB (publ) (354010589) Establishment Name Address ID/FEI Business Operations Patheon Italia S.p.A 434078638 MANUFACTURE(66658-501, 66658-505, 66658-510, 66658-522, 66658-523, 66658-524, 66658-525)