METVIXIA - methyl aminolevulinate hydrochloride cream
Galderma Laboratories, L.P.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METVIXIA safely and effectively. See full prescribing information for METVIXIA. METVIXIA® (methyl aminolevulinate) Cream, 16.8% For Topical Use Only Initial U.S. Approval: 2004
INDICATIONS AND USAGEMETVIXIA Cream, a porphyrin precursor, in combination with the Aktilite® CL128 lamp, a narrowband, red light illumination source, is indicated for treatment of thin and moderately thick, non-hyperkeratotic, non-pigmented actinic keratoses of the face and scalp in immunocompetent patients when used in conjunction with lesion preparation in the physician’s office when other therapies are considered medically less appropriate. (1) DOSAGE AND ADMINISTRATIONMETVIXIA Cream is not for ophthalmic, oral or intravaginal use. (2) (2)
DOSAGE FORMS AND STRENGTHSCream, 16.8% ( 3) (3) CONTRAINDICATIONSMETVIXIA Cream is contraindicated in patients with: (4)
WARNINGS AND PRECAUTIONSMETVIXIA Cream is intended for topical use in the physician’s office by physicians only. The recurrence rate of treated lesions is unknown. (5.1) (5) Patients should be cautioned to wear protective clothing after exposure to METVIXIA. (5.2) (5) METVIXIA Cream has demonstrated a high rate of contact sensitization (allergenicity). (5.3) (5) Patients and providers should wear protective eyewear before operating the Aktilite lamp. (5.4) (5) ADVERSE REACTIONSMost common related adverse reactions (incidence greater than 10% and greater than placebo) are erythema; pain, burning and discomfort; pruritus; scabbing, crusting and erosions; edema and exfoliation of the skin. (6) (6)
See 17 for PATIENT COUNSELING INFORMATION. Revised: 06/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
METVIXIA Cream in combination with Aktilite CL128 lamp red light illumination is indicated for treatment of thin and moderately thick, non-hyperkeratotic, non-pigmented actinic keratoses of the face and scalp in immunocompetent patients. This photodynamic therapy should be used in conjunction with appropriate lesion preparation in the physician’s office when other therapies are considered medically less appropriate.
Limitations of Use:
The safety and efficacy have not been established for the treatment of cutaneous malignancies or for skin lesions other than non-hyperkeratotic face and scalp actinic keratoses using PDT with METVIXIA Cream.
The safety and efficacy of METVIXIA Cream have not been established in patients with immunosuppression, porphyria or pigmented actinic keratoses.
METVIXIA Cream has not been tested on patients with inherited or aquired coagulation defects.
Use of METVIXIA Cream without subsequent red light illumination is not recommended.
2 DOSAGE AND ADMINISTRATION
This product is to be used only by physicians in the physician’s office.
METVIXIA Cream is not for ophthalmic, oral or intravaginal use.
Photodynamic therapy (PDT) for non-hyperkeratotic actinic keratoses with METVIXIA Cream is a multi-stage process as described below: Two treatment sessions one week apart should be administered. Not more than one gram (half tube) of METVIXIA Cream should be applied per treatment session. Multiple lesions may be treated during the same treatment session using a total of one gram of METVIXIA Cream. Lesion response should be assessed 3 months after the last treatment session. Nitrile gloves should be worn when applying and removing the cream.
The Aktilite CL128 lamp, which is equipped with light emitting diodes (LEDs), emits red light with a narrow spectrum at approximately 630 nm, and a half-width of approximately 20 nm. The light dose to be used is 37 J/cm2, and the lamp should be placed 50 to 80 mm from the skin. The area of skin that can be illuminated is 80 x 180 mm. Calibration by the operator is not needed, and the illumination time is calculated automatically. The LED panel window should be cleaned daily with a slightly moist clean cloth.
If Aktilite red light treatment is interrupted or stopped for any reason, it may be restarted. If the patient for any reason cannot have the red light treatment during the prescribed period after application (the 3 hour timespan), the cream should be rinsed off and the patient should protect the exposed area from sunlight, prolonged or intense light for at least 48 hours.
Physicians should be knowledgeable about photodynamic therapy and familiar with the Aktilite Operators Manual prior to use of METVIXIA Cream.
One METVIXIA-PDT session consists of:
- Lesion preparation [See Dosage and Administration (2.1)]
- Application of METVIXIA Cream [See Dosage and Administration (2.2)]
- Application of occlusive dressing [See Dosage and Administration (2.3)]
- Occlusion for 3 hours [See Dosage and Administration (2.4)]
- Removal of excess cream with saline [See Dosage and Administration (2.5)]
- Positioning Aktilite CL128 lamp [See Dosage and Administration (2.6)]
- Illumination with red light (Aktilite CL128 lamp) [See Dosage and Administration (2.7)]
2.1 Lesion preparation
- Before applying METVIXIA Cream, the surface of the lesions should be prepared with a small dermal curette to remove scales and crusts and roughen the surface of the lesion. This is to facilitate access of the cream and light to all parts of the lesion.

Figure 1A Lesion debriding
- Only nitrile gloves should be worn during this and subsequent steps and Universal Precautions should be taken. Vinyl and latex gloves do not provide adequate protection when using this product.

Figure 1B Lesion debriding
2.2 Application of METVIXIA Cream
- Using a spatula, apply a layer of METVIXIA Cream about 1 mm thick to the lesion and the surrounding 5 mm of normal skin. Do not apply more than one gram (half tube) of METVIXIA Cream per treatment session.
 Figure 2: Cream application
Figure 2: Cream application
2.3 Occlusive Dressing Cover
- The area where the cream has been applied should then be covered with an occlusive, non-absorbent dressing for 3 hours. Multiple lesions may be treated during the same treatment session. Each treatment field is limited to an area of 80 x 180 mm.
 Figure 3: Occlusive dressing application
Figure 3: Occlusive dressing application
2.4 Occlusion for 3 hours
(at least 2.5 hours, but no more than 4 hours)
- After Cream application, patients should avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination lamps, operating room lamps, tanning beds, or lights at close proximity) during the period prior to Aktilite red light treatment. Exposure to light may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions. Patients should protect treated areas from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material. Sunscreens will not protect against photosensitivity reactions caused by visible light. It has not been determined if perspiration can spread the METVIXIA Cream outside the treatment site to the eyes or surrounding skin. The treated site should be protected from extreme cold with adequate clothing or remaining indoors between application of METVIXIA Cream and Aktilite PDT light treatment.
2.5 Removal of Excess Cream with Saline
- Following removal of the occlusive dressing, clean the area with saline and gauze. Wear nitrile gloves.
 Figure 4: Cream removal
Figure 4: Cream removal
2.6 Positioning Aktilite CL128 Lamp
- See Aktilite CL128 Operators Manual for specific warnings, cautions and instructions. If necessary adjust the dose to 37 J/cm2 . Calibration by the operator is not required. Position the lamp over the area to be illuminated by the use of guide light. The distance between the LED panel and the lesion surface should be 50 to 80 mm (2 to 3.2 in). Do not stare into the beam. The patient and operator should wear appropriate eye protection during illumination. Patient protective goggles or eye shields are dark or of metal to block visible light.
 Figure 5: Positioning Aktilite CL128
Figure 5: Positioning Aktilite CL128
2.7 Illumination with Aktilite CL128 Lamp Red Light
- The required illumination time (7-10 minutes) is calculated automatically, and remaining time will be displayed at the control panel. The illumination stops automatically. The illumination may be paused and started again. Patients should be advised that transient pain, burning or stinging at the target lesion sites may occur during the period of light exposure.
 Figure 6: Illumination
Figure 6: Illumination
3 DOSAGE FORMS AND STRENGTHS
Cream, 16.8%.
Each gram of METVIXIA Cream contains 168 mg of methyl aminolevulinate in an off-white to pale yellow cream base.
4 CONTRAINDICATIONS
METVIXIA Cream is contraindicated in patients with cutaneous photosensitivity, or known allergies to porphyrins, and in patients with known sensitivities to any of the components of METVIXIA Cream, which includes peanut and almond oil [See WARNINGS and PRECAUTIONS ( 5)]
5 WARNINGS AND PRECAUTIONS
5.1 General
METVIXIA Cream is intended for topical use in the physician’s office by physicians. METVIXIA Cream has not been studied for more than one course which consists of two treatment sessions one week apart.
Clinical studies did not follow patients beyond 3 months, and the recurrence rate of treated lesions is unknown.
5.2 Photosensitivity
During the time period between the application of METVIXIA Cream and exposure to Aktilite red light illumination, the treatment site will become photosensitive.
If for any reason the patient cannot have the Aktilite red light treatment after application of METVIXIA Cream, the cream should be rinsed off, and the patient should protect the treated area from sunlight, prolonged or intense light for two days. Prolonged exposure for greater than 4 hours to METVIXIA Cream should be avoided.
After METVIXIA Cream application, patients should avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination lamps, operating room lamps, tanning beds, or lights at close proximity) during the period prior to red light treatment. Exposure to light may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions. Before exposure to sunlight, patients should, therefore, protect treated lesions from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material. Sunscreens will not protect against photosensitivity reactions caused by visible light. The treated site should be protected from extreme cold with adequate clothing or remaining indoors between application of METVIXIA Cream and Aktilite red light treatment.
After illumination of METVIXIA Cream, the area treated should be kept covered and away from light for at least 48 hours.
Because of the potential for skin to become photosensitized, the METVIXIA Cream should be used by a trained physician to apply drug only to non-hyperkeratotic actinic keratoses and perilesional skin within 5 mm of the lesion. Redness, swelling, burning, and stinging are expected as a result of therapy; however, if these symptoms increase in severity and persist longer than 3 weeks, the patient should contact their doctor.
5.3 Hypersensitivity
METVIXIA Cream has demonstrated a high rate of contact sensitization (allergenicity) [See Adverse Reactions (6.2)]. Care should be taken by the physician applying METVIXIA Cream to avoid inadvertent skin contact. Nitrile gloves should be worn when applying and removing the cream. Vinyl and latex gloves do not provide adequate protection when using this product.
METVIXIA Cream is formulated with peanut oil and refined almond oil.
METVIXIA Cream has not been tested in patients who are allergic to peanuts.
5.4 Aktilite Lamp
Before operating the Aktilite CL128 lamp, personnel should refer to the Operators Manual for specific warnings, cautions and instructions. Care should be exercised when positioning and operating the lamp. During the red light illumination period, the patient, operator and other persons present should wear protective goggles that sufficiently screen out the appropriate spectrum of red light. The protective goggles or eye shields provided for the patient are dark or of metal to block visible light. The green professional protective glasses provided for the operator screen out the relevant spectrum of red light and the room will still appear bright for the operator to see. Do not stare into the beam. For lamp assembly, maintenance, service and technical data the personnel should refer to the Operators Manual.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 231 subjects, each with 4 - 10 actinic keratoses were enrolled in 2 double-blind, randomized, vehicle-controlled clinical trials. Subjects were randomized to receive Aktilite PDT with METVIXIA Cream 16.8 % or Vehicle cream on 2 occasions 1 week apart. Cream was applied for approximately 3 hours under occlusion followed immediately by illumination using the Aktilite CL128 lamp, delivering red light at a dose of 37 J/cm2.
Table 1 shows the incidence and severity of local (treatment site) adverse reactions in these two trials. The most frequent adverse reactions were associated with phototoxicity at the treatment site. Pain and burning sensation typically begin during illumination and generally resolve completely within a few minutes or hours, but may last up to a few days. Erythema and other signs generally resolve within a few days to 3 weeks.
In these two trials, out of 126 subjects treated with METVIXIA Cream, six METVIXIA Aktilite PDT subjects did not complete the full two treatment session regimen due to adverse reactions such as headache, pain or burning. These subjects either stopped illumination early or did not have the second treatment. In addition, 12 METVIXIA PDT subjects paused illumination due to pain, burning or stinging but did subsequently complete treatment.
| METVIXIA & Aktilite PDT n=126 | Vehicle & Aktilite PDT n=105 |
|||
| All Grades* | Severe | All Grades* | Severe | |
| Any Treatment Site Adverse Reaction | 113 (90%) | 28 (22%) | 48 (46%) | 0 (0%) |
| Skin burning/pain/discomfort | 109 (86%) | 25 (20%) | 38 (36%) | 0 (0%) |
| Erythema | 80 (63%) | 7 (6%) | 11 (10%) | 0 (0%) |
| Scabbing/crusting/blister/erosions | 36 (29%) | 2 (2%) | 1 (1%) | 0 (0%) |
| Pruritus | 28 (22%) | 0 (0%) | 8 (8%) | 0 (0%) |
| Skin or eyelid edema | 23 (18%) | 2 (2%) | 1 (1%) | 0 (0%) |
| Skin exfoliation | 17 (14%) | 4 (3%) | 3 (3%) | 0 (0%) |
| Skin warm | 5 (4%) | 0 (0%) | 2 (2%) | 0 (0%) |
| Application site discharge | 3 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Skin hemorrhage | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Skin tightness | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Skin hyperpigmentation | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
* Mild, Moderate, or Severe
6.2 Dermal Safety Studies
Studies in healthy volunteer subjects and subjects with actinic keratoses previously treated with METVIXIA-PDT on at least 4 previous occasions have demonstrated that METVIXIA Cream has the potential to cause irritancy and sensitization. A cumulative irritancy and sensitization (allergenicity) study of METVIXIA Cream with a cross-sensitization challenge with aminolevulinic acid (ALA) was performed in 156 subjects. METVIXIA Cream was applied 3 times each week for 3 weeks (total of 9 applications), to separate sites on the back of healthy volunteers. After each application, the area was covered by aluminum Finn Chamber. After the 3-week continuous treatment period and a 2-week interval without further applications, subjects were challenged with METVIXIA Cream, METVIXIA vehicle, ALA, and ALA-vehicle creams for 48 hours. Assessment of skin reactions was performed 48, 72, and 96 h after start of the challenge cream application. Only 98 of the 156 subjects tested entered the challenge phase because of a high incidence of local irritancy evident as erythema. Of the 58 subjects who were challenged with METVIXIA Cream, 30 (52 %) showed contact sensitization. Of the 98 subjects who were challenged with ALA, only 2 (2 %) showed equivocal reactions the remaining subjects having negative responses.
The potential for sensitization was also assessed by patch testing a total of 21 patients with actinic keratoses previously treated with METVIXIA-PDT on at least 4 previous occasions. METVIXIA Cream 16.8 % and vehicle cream were applied to different sites on the lower back for 48 hours. Three of the 21 patients (14%) showed contact sensitization associated with erythema scores ≥4 (strong erythema spreading outside the patch) and edema, vesiculation, papules and glazing.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post approval use of METVIXIA Cream. Because these reactions are reported voluntary from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic reactions reported include eczema, allergic contact dermatitis and urticaria. Most cases were localized to the treatment area; rarely erythema and swelling have been more extensive. At sites distant from the application site there have been reports of squamous cell carcinoma of the skin, as expected in this population. There have been occasional reports of eye disorders including macular edema, vitreous detachment and keratitis. There have been reports of angioedema, facial edema and application site infections.
7 DRUG INTERACTIONS
There have been no studies of the interaction of METVIXIA Cream with other drugs, including local anesthetics. It is possible that concomitant use of other known photosensitizing agents might increase the photosensitivity reaction of actinic keratoses treated with METVIXIA Cream.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects: Pregnancy Category C:
There are no adequate and well-controlled studies with METVIXIA Cream in pregnant women. Intravenous methyl aminolevulinate hydrochloride (HCI) was teratogenic in rabbits at a high dose. METVIXIA Cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
A Maximum Topical Human Dose (MTHD) of 2 g of METVIXIA Cream 16.8% containing 420 mg methyl aminolevulinate hydrochloride corresponding to 7 mg/kg or 259 mg/m2 for a 60 kg patient and an estimated maximum systemic uptake of 1% was used for the animal multiple of human systemic exposure calculations presented in this labeling.
In development toxicity studies, pregnant rabbits received intravenous doses of methyl aminolevulinate hydrochloride up to 926 times the MTHD on Days 6 to 18 of gestation. Slightly lower fetal body weights and increased incidences of fetuses with jugals connected/fused to maxilla, supernumerary ribs, incompletely ossified cranial bones and other ossification irregularities were noted in the high dose (926 times the MTHD) group, compared to the control group. The embryo-fetal effects in the high dose group were associated with maternal toxicity. These effects did not occur at 463 times the MTHD based on mg/m2 comparisons and an estimated maximum systemic uptake of 1%. Developmental toxicity studies in rats were negative at daily exposure levels up to 1622 times the MTHD on a mg/m2 basis.
In the prenatal and postnatal development toxicity study, pregnant rats received intravenous doses of methyl aminolevulinate hydrochloride up to 1160 x the MTHD from Day 6 of gestation to Day 24 of lactation. There were no treatment-related effects on litter size, pup mortality, pup weights, or post weaning performance in the pups (including development and reproduction). A slightly longer duration of gestation and a slight delay in pup physical development were noted in the 580-1160 x the MTHD groups [See Nonclinical Toxicology (13.2)].
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when METVIXIA Cream is administered to a nursing woman.
8.4 Pediatric Use
Actinic keratosis is not a condition generally seen within the pediatric population. The safety and effectiveness in pediatric patients below the age of 18 have not been established.
8.5 Geriatric Use
Of the 211 subjects in the clinical studies with METVIXIA-PDT, 136 subjects were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
10 OVERDOSAGE
10.2 Aktilite Red Light Overdose
Red light overdose (excess illumination time) using Aktilite CL128 following METVIXIA Cream application has not been reported. If red light overexposure were to result in a burn, the patient should be treated in accordance with standard practice for treatment of cutaneous burns.
11 DESCRIPTION
METVIXIA (methyl aminolevuliante) Cream is an oil in water emulsion. METVIXIA Cream contains methyl aminolevulinate hydrochloride equivalent to 168 mg/g of methyl aminolevulinate.
Methyl aminolevulinate hydrochloride is a white to slightly yellow powder that is freely soluble in water and methanol, soluble in ethanol and practically insoluble in most organic solvents.
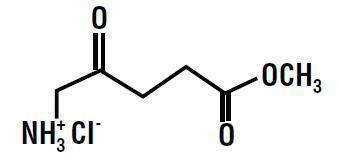
The chemical formula for methyl aminolevulinate hydrochloride is C6H11NO3•HCl (MW=181.62). The chemical name for methyl aminolevulinate hydrochloride is methyl 5-amino-4-oxopentanoate hydrochloride and it has the following structural formula:
METVIXIA Cream is off-white to pale yellow in color, and contains the following excipients: cetostearyl alcohol, cholesterol, edentate disodium, glycerin, isopropyl myristate, oleyl alcohol, peanut oil, polyoxyl 40 stearate, purified water, refined almond oil, self-emulsifying glyceryl monostearate, white petrolatum, and methylparaben and propylparaben as preservatives.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Photosensitization following application of METVIXIA Cream occurs through the metabolic conversion of methyl aminolevulinate (prodrug) to photoactive porphyrins (PAPs), which accumulate in the skin lesions to which METVIXIA Cream has been applied. When exposed to light of appropriate wavelength and energy, the accumulated photoactive porphyrins produce a photodynamic reaction, resulting in a cytotoxic process dependent upon the simultaneous presence of oxygen. The absorption of light results in an excited state of porphyrin molecules, and subsequent spin transfer from photoactive porphyrins to molecular oxygen generates singlet oxygen. METVIXIA photodynamic therapy (PDT) of actinic (solar) keratosis lesions is the combination of photosensitization by topical application of METVIXIA Cream to the lesions and subsequent illumination with red light of narrow spectrum using a light dose of 37 J/cm2 delivered by the Aktilite CL128 lamp.
12.3 Pharmacokinetics
The time-course of Protoporphyrin IX in actinic keratosis lesions and surrounding skin after application of METVIXIA Cream has been monitored by means of fluorescence. The optimum concentration of methyl aminolevulinate cream (16.8 %) and duration of application (3 h) were derived from such studies of pharmacokinetics in skin using a range of concentrations (1.6%, 8% and 16.8%) and cream application times (up to 28 h). Three hours after the application of METVIXIA Cream fluorescence in the treated lesions was significantly greater than that seen in both treated and untreated normal skin, and after application of vehicle cream (not containing methyl aminolevulinate) to normal skin. In a fluorescence study of 8 patients with actinic keratoses using METVIXIA Cream 16.8% applied for 3 h and illumination with the Aktilite CL128 lamp, 88% photodegradation of Protoporphyrin IX was observed immediately after illumination, followed by a transient small secondary increase in fluorescence 2 hours after illumination. At 24 and 48 hours, 94% and 96% degradation of Protoporphyrin IX, respectively from baseline, was observed.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of METVIXIA Cream have not been performed. Methyl aminolevulinate was negative for genetic toxicity in the Ames assay, and the chromosomal aberration assay in Chinese hamster ovary cells, tested with and without metabolic activation and in the presence and absence of light. Methyl aminolevulinate was also negative in the in vivo micronucleus assay in the rat. In contrast, at least one report in the literature has noted genotoxic effects in cultured rat hepatocytes after aminolevulinate (ALA) exposure with PpIX formation. Other studies have documented oxidative DNA damage in vivo and in vitro as a result of ALA exposure.
A fertility study was performed in male and female rats with intravenous doses of methyl aminolevulinate hydrochloride up to 500 mg/kg/day (3000 mg/m2, 1158 times the MTHD based on mg/m2 comparisons and an estimated maximum systemic uptake of 1%). Males were treated for 4 weeks prior to mating and for 5 additional weeks after mating. The females were treated for 2 weeks prior to mating and then until Day 6 of gestation. There were no treatment-related effects on fertility and mating performance seen in this study.
13.2 Reproductive Toxicology
Development toxicity studies have been performed in pregnant rats with intravenous doses of methyl aminolevulinate hydrochloride up to 700 mg/kg/day on Days 6 to 16 of gestation. There were no treatment-related effects on fetal body weight, sex ratio, external malformations and variations, and skeletal abnormalities and ossification extent. Only a slight non-significant increase in early embryonic death was noted in the 700 mg/kg/day group, compared to the control group. The fetal NOAEL (No Adverse Effect Level) was 350 mg/kg/day methyl aminolevulinate hydrochloride in pregnant rats (2100 mg/m2, 811 times the MTHD based on mg/m2 comparisons and an estimated maximum systemic uptake of 1%).
Development toxicity studies have also been performed in pregnant rabbits with intravenous doses of methyl aminolevulinate hydrochloride up to 200 mg/kg/day on Days 6 to 18 of gestation. Slightly lower fetal body weights and increased incidences of fetuses with jugals connected/fused to maxilla, supernumerary ribs, incompletely ossified cranial bones and other ossification irregularities were noted in the high dose (200 mg/kg/day) group, compared to the control group. The fetal NOAEL was 100 mg/kg/day methyl aminolevulinate hydrochloride in pregnant rabbits (1200 mg/m2, 463 times the MTHD based on mg/m2 comparisons and an estimated maximum systemic uptake of 1%).
In the prenatal and postnatal development toxicity study in rats treated with intravenous doses of methyl aminolevulinate hydrochloride up to 500 mg/kg/day from Day 6 of gestation to Day 24 of lactation, there were no treatment-related effects on litter size, pup mortality, pup weights, and post weaning performance of the F1 animals including development and reproductive capacity. Only a slightly longer duration of gestation and a slight delay in pup physical development were noted in the 250 and 500 mg/kg/day groups. The NOAEL was 125 mg/kg/day methyl aminolevulinate hydrochloride (750 mg/m2, 290 times the MTHD based on mg/m2 comparisons and an estimated maximum systemic uptake of 1%).
14 CLINICAL STUDIES
METVIXIA Cream 16.8 % for photodynamic therapy (PDT) by illumination using the Aktilite CL128 lamp was studied in 211 randomized subjects with a total of 1555 non-hyperkeratotic actinic keratoses in two multicenter, randomized, double-blind vehicle-controlled clinical trials. One trial was conducted in the USA and the other in the USA and Germany.
Each subject had 4 to 10 previously untreated, nonpigmented, grade 1 (thin) or 2 (moderate) actinic keratoses on the face and or scalp. Grade 3 (very thick and obvious) actinic keratoses were not treated in the trials. Two sessions of PDT were administered at an interval of one week with patients randomized 1:1 to receive METVIXIA-PDT or Vehicle-PDT on both occasions. Each session comprised lesion preparation (debridement with sharp curette) to roughen the surface, application of cream with subsequent maintenance for 3 hours under occlusion using an adhesive, non-absorbent dressing, removal of residual cream followed immediately by light activation. Red light illumination (630 nm) was provided by the Aktilite CL128 lamp and the light dose was 37 J/cm2.
The subject complete response rate was assessed 3 months after the last treatment. Lesion clinical complete response was defined as complete disappearance of a lesion upon visual inspection and palpation. If all treated lesions within a subject were in clinical complete response 3 months after treatment, the subject was assessed as a complete responder. The subject complete response rates are shown in Table 2 for each of the two studies.The overall lesion complete response rates and the response rates by lesion grade and location are shown in Table 3 for the two studies.
| Study 1 | Study 2 | ||||
|
METVIXIA n=49 |
Vehicle n=47 |
METVIXIA n=57 |
Vehicle n=58 |
||
| Subjects with Complete Response | 29 | 7 | 39 | 4 | |
| 59.2% | 14.9% | 68.4% | 6.9% | ||
| Study 1 | Study 2 | ||||
| METVIXIA N=363 | Vehicle n=360 | METVIXIA n=418 | Vehicle n=414 |
||
| Lesions with Complete response | |||||
| Grade 1 | 259 | 267 | 182 | 161 | |
| Face | Total | 191 | 201 | 99 | 88 |
| Face | CR | 167 (87%) | 121 (60%) | 90 (91%) | 33 (38%) |
| Scalp | Total | 68 | 66 | 76 | 73 |
| Scalp | CR | 63 (93%) | 29 (44%) | 66 (87%) | 31 (42%) |
|
Grade 2 | 104 | 93 | 236 | 253 | |
| Face | Total | 76 | 68 | 119 | 157 |
| Face | CR | 65 (86%) | 29 (43%) | 103 (87%) | 35 (22%) |
| Scalp | Total | 28 | 25 | 115 | 96 |
| Scalp | CR | 18 (64%) | 9 (36%) | 89 (77%) | 20 (21%) |
There was no difference between response rates to METVIXIA Aktilite PDT for Grade 1 lesions on the face and scalp (CR rates of 89 % and 90 % respectively). For Grade 2 lesions, the corresponding CR rates to METVIXIA Aktilite PDT were 86 % and 75 % respectively. METVIXIA Cream has not been studied for more than one course which consists of two treatment sessions one week apart. There is no information regarding the recurrence rate for lesions treated with this therapy. Clinical studies did not follow patients beyond 3 months, and the recurrence rate of treated lesions is unknown.
16 HOW SUPPLIED/STORAGE AND HANDLING
METVIXIA Cream, 16.8%, is available as the following:
2 gram aluminum tube, box of 1 (NDC 0299-6300-02)
Keep out of reach of children
For topical use only by physicians in the physician’s office. Dispense only to physicians and only to be applied by a physician.
Physicians should wear nitrile gloves when applying and removing METVIXIA Cream. Vinyl and latex gloves do not provide adequate protection when using this product.
Store/Transport refrigerated, 2˚ - 8˚C (36˚ - 46˚F).
Use contents within one week after opening.
Should not be used after 24 hours out of refrigerator.
17 PATIENT COUNSELING INFORMATION
See FDA Approved Patient Labeling (Patient Information)
The physician should discuss the following with each patient.
PATIENT INFORMATION
METVIXIA (met vik see a)
(methyl aminolevulinate hydrochloride) Cream, 16.8%
Read this Patient Information before you are treated with METVIXIA Cream and each time you are treated. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or treatment. Ask your doctor provider about anything you do not understand about METVIXIA Cream.
Important note: METVIXIA Cream is for use on the skin only. Tell your doctor right away if METVIXIA Cream gets in your eyes or mouth.
What is the most important thing I need to know about METVIXIA Cream?
- METVIXIA Cream with light treatment (Photodynamic therapy or PDT) is only done in medical offices by trained doctors.
- METVIXIA Cream is not applied by patients and should not be applied by doctors who have not been trained in its use.
- METVIXIA Cream is for use in PDT with the Aktilite CL128 lamp.
What is METVIXIA Cream?
- METVIXIA Cream is a prescription cream used with PDT to treat skin growths on the face and scalp called actinic keratoses (AK).
- METVIXIA Cream is only used for AK skin growths that are thin and not dark colored. AK skin growths are caused partly by too much sun exposure. METVIXIA Cream and PDT work together to treat AK skin growths.
- METVIXIA Cream has not been studied in children for any condition and should not be used in children.
Who should not use METVIXIA Cream?
Do not use METVIXIA Cream if:
- your skin over reacts to sun or light (photosensitivity). Talk with your doctor to be sure.
- you are allergic to porphyrins or to any of the ingredients in METVIXIA Cream including peanut and almond oil. See the end of this leaflet for a complete list of ingredients in METVIXIA Cream.
What should I tell my doctor before treatment with METVIXIA Cream?
Tell your doctor about all your medical conditions, including if you
- have or had skin cancer or other skin growths on your body
- have bleeding problems
- are pregnant or planning to become pregnant. It is not known if METVIXIA Cream can harm your unborn baby.
- are breastfeeding. It is not known if METVIXIA Cream passes into your milk and if it can harm your baby. You should decide whether or not to stop breastfeeding while getting treatment with METVIXIA Cream. Talk to your doctor for help with this choice.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. It is not known if METVIXIA Cream and other medicines can affect each other.
Know your medicines. Keep a list of your medicines and show it to your doctor and pharmacist when you start a new medicine.
How should I use METVIXIA Cream?
- METVIXIA Cream and PDT treatment is only done by trained doctors.
- You will receive 2 treatments with METVIXIA Cream and PDT 1 week apart.
- Your doctor will check you three months after treatment to see if the treatment worked for you. (See the end of this leaflet for the section “Treatment with METVIXIA Cream and PDT.”)
- METVIXIA Cream is for skin use only. Do not get METVIXIA Cream in your eyes or mouth. Tell your doctor right away if this happens.
What should I avoid while using METVIXIA Cream?
During the 3 hours that METVIXIA Cream is on your skin:
- Avoid sunlight or bright indoor light (for example: examination lights, operating room lights, tanning beds, or lights that are close to you.) During this time, the treated areas of your skin are more sensitive to light. You may feel burning or stinging. Your treated skin may become red or your lesions may become swollen. Wear a protective hat and clothing if you need to be outside in the sun.
- Avoid cold temperatures. Wear warm clothing and keep your treated skin areas covered if you are in cold temperatures, or stay indoors.
- After treatment with METVIXIA Cream and PDT, avoid sunlight or bright indoor light (for example: examination lights, operating room lights, tanning beds or lights that are close to you) for two days. During this time, the treated areas of your skin are more sensitive to light. Keep the treated areas of your skin covered. Wear a protective hat and clothing if you need to be outside in the sun.
- If for any reason you are not treated with the lamp after METVIXIA Cream has been applied to your skin:
- Carefully rinse off the Cream.
- Avoid sunlight, indoor light that is bright (for example: examination lights, operating room lights, tanning beds or lights that are close to you) for two days after treatment. During this time, the treated areas of your skin are more sensitive to light. Wear a protective hat and clothing if you need to be outside in the sun.
What are the possible side effects of METVIXIA Cream with PDT treatment?
Common side effects of METVIXIA Cream with PDT treatment include the following skin reactions at the treated site:
- redness
- pain
- burning feeling
- stinging
- swelling
- crusting, peeling, blisters, bleeding, itching ulcers
Burning pain and stinging usually decline over a few hours. If skin redness, swelling and other signs of inflammation get worse and last longer than 3 weeks, call your doctor.
Tell your doctor about any side effects that bother you or do not go away. Your doctor should be able to treat them if needed.
These are not all the side effects of METVIXIA Cream with PDT. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about METVIXIA Cream
This leaflet summarizes the most important information about METVIXIA Cream. If you would like more information, talk with your doctor. You can ask your doctor for information about METVIXIA Cream that is written for health professionals.
What are the ingredients in METVIXIA Cream?
Active Ingredient: methyl aminolevulinate hydrochloride.
Inactive Ingredients: Glyceryl monostearate, cetostearyl alcohol, poloxyl stearate, cholesterol, oleyl alcohol glycerin, white petrolatum, isopropyl myristate, peanut oil, refined almond oil, edetate disodium, methylparaben and propylparaben.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Manufactured by: Penn Pharmaceutical Services Ltd., Tafamaubach Industrial Estate, Tredegar, Gwent, NP22 3AA, UK
Made in United Kingdom.
PACKAGE LABEL
metvixia®
(methyl aminolevulinate) Cream, 16.8%
CREAM
NDC 0299-6300-02
Rx only
NET WT. 2 g
GALDERMA
For topical use only. Must be dispensed and applied by a physician.
For use with an Aktilite® CL 128 lamp only.
Each gram contains 210 mg of methyl aminolevulinate hydrochloride (equiv. to 168 mg methyl aminolevulinate), cetostearyl alcohol, cholesterol, edetate disodium, glycerin, isopropyl myristate, methylparaben, oleyl alcohol, peanut oil, polyoxyl 40 stearate, propylparaben, purified water, refined almond oil, self-emulsifying glyceryl monostearate, and white petrolatum.
Directions for use: See enclosed package insert. Use contents within one week after opening. Keep out of reach of children.
Should not be used after 24 hours out of refrigerator.
Storage Conditions: Store/Transport refrigerated, 2° - 8° C (36° - 46°F).
See carton closure for lot number and expiration date.
Distributed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
P24203-1
Manufactured by:
Penn Pharmaceutical Services Ltd.
Tredegar, Gwent NP22 3AA, UK
Made in United Kingdom
Trademarks are the property of their respective owners.
Lot No.:
Exp. date:
| METVIXIA
methyl aminolevulinate cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| PENN PHARMACEUTICAL SERVICES LTD | 226277259 | manufacture(0299-6300) | |