LEVO-DROMORAN- levorphanol tartrate injection, solution
LEVO-DROMORAN- levorphanol tartrate tablet
Valeant Pharmaceuticals North America LLC
----------
LEVO-DROMORAN™ C-II
(levorphanol tartrate)
AMPULS, VIALS, TABLETS
Potent Synthetic Opioid
For Parenteral Or Oral Administration
DESCRIPTION
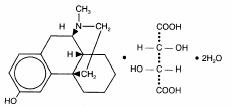
Levo-Dromoran (levorphanol tartrate) is a potent opioid analgesic with empirical formula C17H23NO•C4H6O6•2H2O and molecular weight 443.5. Each mg of levorphanol tartrate is equivalent to 0.58 mg levorphanol base. Chemically levorphanol is levo-3-hydroxy-N-methylmorphinan. The USP nomenclature is 17-methylmorphinan 3-ol tartrate (1:1)(Salt) dihydrate. The material has 3 asymmetric carbon atoms. The chemical structure is:
Levorphanol tartrate is a white crystalline powder, soluble in water and ether but insoluble in chloroform.
Each 1-mL ampul contains 2 mg levorphanol tartrate, 1.8 mg methyl paraben preservative, 0.2 mg propyl paraben preservative, sodium hydroxide to adjust pH to approximately 4.3 and Water for Injection.
Each milliliter in the 10 mL vials contains 2 mg levorphanol tartrate, 4.5 mg phenol preservative, sodium hydroxide to adjust pH to approximately 4.3 and Water for Injection.
Each tablet contains 2 mg levorphanol tartrate, lactose, corn starch, stearic acid and talc.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Levo-Dromoran is a potent synthetic opioid similar to morphine in its actions. Like other mu-agonist opioids it is believed to act at receptors in the periventricular and periaqueductal gray matter in both the brain and spinal cord to alter the transmission and perception of pain. Onset of analgesia and peak analgesic effect following administration of levorphanol are similar to morphine when administered at equianalgesic doses.
Levorphanol produces a degree of respiratory depression similar to that produced by morphine at equianalgesic doses, and like many mu-opioid drugs, levorphanol produces euphoria or has a positive effect on mood in many individuals. Two mg of intramuscular levorphanol tartrate depresses respiration to a degree approximately equivalent to that produced by 10 to 15 mg of intramuscular morphine in man. The hemodynamic changes after intravenous administration of levorphanol have not been studied in man but are expected to clinically resemble those seen after morphine.
As with other opioids, the blood levels required for analgesia are determined by the opioid tolerance of the patient and are likely to rise with chronic use. The rate of development of tolerance is highly variable and is determined by the dose, dosing interval, age, use of concomitant drugs and physical status of the patient. While blood levels of opioid drugs may be helpful in assessing individual cases, dosage is usually adjusted by careful clinical observation of the patient.
Pharmacokinetics
The pharmacokinetics of levorphanol have been studied in a limited number of cancer patients following intravenous (IV), intramuscular (IM) and oral (PO) administration. Following IV administration, plasma concentrations of levorphanol decline in a triexponential manner with a terminal half-life of approximately 11 to 16 hours and a clearance of 0.78 to 1.1 L/kg/hr. Based on terminal half-life, steady-state plasma concentrations should be achieved by the third day of dosing. Levorphanol is rapidly distributed (<1 hr) and redistributed (1 to 2 hours) following IV administration and has a steady-state volume of distribution of 10 to 13 L/kg. In vitro studies of protein binding indicate that levorphanol is only 40% bound to plasma proteins.
No pharmacokinetic studies of the absorption of IM levorphanol are available, but clinical data suggests that absorption is rapid with onset of effects within 15 to 30 minutes of administration.
Levorphanol is well absorbed after PO administration with peak plasma concentrations occurring approximately 1 hour after dosing. The bioavailability of levorphanol tablets compared to IM or IV administration is not known.
Plasma concentrations of levorphanol following chronic administration in patients with cancer increased with the dose, but the analgesic effect was dependent on the degree of opioid tolerance of the patient. Expected steady-state plasma concentrations for a 6-hour dosing interval can reach 2 to 5 times those following a single dose, depending on the patient’s individual clearance of the drug. Very high plasma concentrations of levorphanol can be reached in patients on chronic therapy due to the long half-life of the drug. One study in 11 patients using the drug for control of cancer pain reported plasma concentrations from 5 to 10 ng/mL after a single 2-mg dose up to 50 to 100 ng/mL after repeated oral doses of 20 to 50 mg/day.
Animal studies suggest that levorphanol is extensively metabolized in the liver and is eliminated as the glucuronide metabolite. This renally excreted inactive glucuronide metabolite accumulates with chronic dosing in plasma at concentrations that reach fivefold that of the parent compound.
The effects of age, gender, hepatic and renal disease on the pharmacokinetics of levorphanol are not known. As with all drugs of this class, patients at the extremes of age are expected to be more susceptible to adverse effects because of a greater pharmacodynamic sensitivity and probable increased variability in pharmacokinetics due to age or disease.
CLINICAL TRIALS
Clinical trials have been reported in the medical literature that investigated the use of Levo-Dromoran as a preoperative medication, as a postoperative analgesic and in the management of chronic pain due primarily to malignancy. In each of these clinical settings Levo-Dromoran has been shown to be an effective analgesic of the mu-opioid type and similar to morphine, meperidine or fentanyl.
A single 2 mg intramuscular dose of Levo-Dromoran was studied as a routine preoperative medication in 100 patients as part of a blinded 1500 patient trial of a number of synthetic opioids and was found to provide sedation similar to that observed with 100 mg meperidine or 10 mg of methadone.
Levo-Dromoran has been studied in chronic cancer patients. Dosages were individualized to each patient’s level of opioid tolerance. In one study, starting doses of 2 mg twice a day often had to be advanced by 50% or more within a few weeks of starting therapy. A study of levorphanol indicates that the relative potency is approximately 4 to 8 times that of morphine, depending on the specific circumstances of use. In postoperative patients, intramuscular levorphanol was determined to be about 8 times as potent as intramuscular morphine, whereas in cancer patients with chronic pain, it was found to be only about 4 times as potent.
INDIVIDUALIZATION OF DOSAGE
Accepted medical practice dictates that the dose of any opioid analgesic be appropriate to the degree of pain to be relieved, the clinical setting, the physical condition of the patient, and the kind and dose of concurrent medication. This is especially important during recovery from anesthesia because of the residual CNS-depressant effects of anesthetic agents and the adverse effects of surgery on respiratory reserve. In consequence, the dose of Levo-Dromoran should be reduced under circumstances likely to increase the patient’s sensitivity to the adverse effects of opioids. As there is substantial redistribution involved in the kinetics of levorphanol, the duration of effect of a single dose may vary and physicians must judge the need for a repeat dose based on the clinical response of the patient. Clinicians are advised to remember that while the long terminal half-life of levorphanol may reduce the need for postoperative analgesics, the administration of an excessive dose preoperatively may cause a delay in the return of spontaneous respirations or prolonged hypoventilation in the postoperative period. In addition, accumulation of the drug following excessive dosage postoperatively may prolong or result in hypoventilation.
Levo-Dromoran has a long half-life similar to methadone or other slowly excreted opioids, rather than quickly excreted agents such as morphine or meperidine. Slowly excreted drugs may have some advantages in the management of chronic pain. Unfortunately, the duration of pain relief after a single dose of a slowly excreted opioid cannot always be predicted from pharmacokinetic principles, and the inter-dose interval may have to be adjusted to suit the patient’s individual pharmacodynamic response.
Levo-Dromoran is 4 to 8 times as potent as morphine and has a longer half-life. Because there is incomplete cross-tolerance among opioids, when converting a patient from morphine to Levo-Dromoran, the total daily dose of oral Levo-Dromoran should begin at approximately 1/15 to 1/12 of the total daily dose of oral morphine that such patients had previously required and then the dose should be adjusted to the patient’s clinical response. If a patient is to be placed on fixed-schedule dosing (round-the-clock) with this drug, care should be taken to allow adequate time after each dose change (approximately 72 hours) for the patient to reach a new steady-state before a subsequent dose adjustment to avoid excessive sedation due to drug accumulation.
INDICATIONS AND USAGE
Levo-Dromoran is indicated for the management of moderate to severe pain or as a preoperative medication where an opioid analgesic is appropriate.
CONTRAINDICATIONS
Levo-Dromoran is contraindicated in patients hypersensitive to levorphanol tartrate.
WARNINGS
Respiratory Depression
Levo-Dromoran, like morphine, may be expected to produce serious or potentially fatal respiratory depression if given in an excessive dose, too frequently, or if given in full dosage to compromised or vulnerable patients. This is because the doses required to produce analgesia in the general clinical population may cause serious respiratory depression in vulnerable patients. Safe usage of this potent opioid requires that the dose and dosage interval be individualized to each patient based on the severity of the pain, weight, age, diagnosis and physical status of the patient, and the kind and dose of concurrently administered medication.
The initial dose of Levo-Dromoran should be reduced by 50% or more when the drug is given to patients with any condition affecting respiratory reserve or in conjunction with other drugs affecting the respiratory center. Subsequent doses should then be individually titrated according to the patient’s response. Respiratory depression produced by levorphanol tartrate can be reversed by naloxone, a specific antagonist (see OVERDOSAGE).
Preexisting Pulmonary Disease
Because Levo-Dromoran causes respiratory depression, it should be administered with caution to patients with impaired respiratory reserve or respiratory depression from some other cause (eg, from other medication, uremia, severe infection, obstructive respiratory conditions, restrictive respiratory diseases, intrapulmonary shunting or chronic bronchial asthma). As with other strong opioids, use of Levo-Dromoran in acute or severe bronchial asthma is not recommended (see Respiratory Depression).
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of Levo-Dromoran with carbon dioxide retention and secondary elevation of cerebral spinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or pre-existing increase in intracranial pressure. Opioids, including Levo-Dromoran, produce effects that may obscure neurological signs of further increase in pressure in patients with head injuries. In addition, Levo-Dromoran may affect level of consciousness that may complicate neurological evaluation.
Cardiovascular Effects
The use of Levo-Dromoran in acute myocardial infarction or in cardiac patients with myocardial dysfunction or coronary insufficiency should be limited because the effects of levorphanol on the work of the heart are unknown.
Hypotensive Effect
The administration of Levo-Dromoran may result in severe hypotension in the postoperative patient or in any individual whose ability to maintain blood pressure has been compromised by a depleted blood volume or by administration of drugs, such as phenothiazines or general anesthetics. Opioids may produce orthostatic hypotension in ambulatory patients.
Use in Liver Disease
Levo-Dromoran should be administered with caution to patients with extensive liver disease who may be vulnerable to excessive sedation due to increased pharmacodynamic sensitivity or impaired metabolism of the drug.
Biliary Surgery
Levo-Dromoran has been shown to cause moderate to marked rises in pressure in the common bile duct when given in analgesic doses. It is not recommended for use in biliary surgery.
Use in Alcoholism or Drug Dependence
Levo-Dromoran has an abuse potential as great as morphine, and the prescription of this drug must always balance the prospective benefits against the risk of abuse and dependence. The use of levorphanol in patients with a history of alcohol or other drug dependence, either active or in remission, has not been specifically studied (see DRUG ABUSE AND DEPENDENCE).
PRECAUTIONS
General
As with other opioids, the administration of Levo-Dromoran may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Levo-Dromoran should be administered with caution and the initial dose should be reduced in patients who are elderly or debilitated and in those patients with severe impairment of hepatic or renal function, hypothyroidism, Addison’s disease, toxic psychosis, prostatic hypertrophy or urethral stricture, acute alcoholism, or delirium tremens.
Information for Patients
If Levo-Dromoran is administered to ambulatory patients, they should be cautioned against engaging in hazardous occupations requiring complete mental alertness such as operating machinery or driving a motor vehicle. They should also be warned that concurrent use of Levo-Dromoran with central nervous system depressants (eg, alcohol, sedatives, hypnotics, other opioids, barbiturates, tricyclic antidepressants, phenothiazines, tranquilizers, skeletal muscle relaxants and antihistamines) may result in additive central nervous system depressant effects. Patients should be made aware of the risk of orthostatic hypotension, dizziness and syncope in ambulatory patients taking Levo-Dromoran.
Drug Interactions
Interactions with Other CNS Agents: Concurrent use of Levo-Dromoran with all central nervous system depressants (eg, alcohol, sedatives, hypnotics, other opioids, general anesthetics, barbitu-rates, tricyclic antidepressants, phenothiazines, tranquilizers, skeletal muscle relaxants and antihistamines) may result in additive central nervous system depressant effects. Respiratory depression, hypotension, and profound sedation or coma may occur. When such combined therapy is contemplated, the dose of one or both agents should be reduced. Although no interaction between MAO inhibitors and Levo-Dromoran has been observed, it is not recommended for use with MAO inhibitors.
Most cases of serious or fatal adverse events involving Levo-Dromoran reported to the manufacturer or the FDA have involved either the administration of large initial doses or too frequent doses of the drug to nonopioid tolerant patients, or the simultaneous administration of levorphanol with other drugs affecting respiration (see INDIVIDUALIZATION OF DOSAGE and WARNINGS). The initial dose of levorphanol should be reduced by approximately 50% or more when it is given to patients along with another drug affecting respiration.
Interactions with Mixed Agonist/Antagonist Opioid Analgesics: Agonist/antagonist analgesics (eg, pentazocine, nalbuphine, butorphanol, dezocine and buprenorphine) should NOT be administered to a patient who has received or is receiving a course of therapy with a pure agonist opioid analgesic such as Levo-Dromoran. In opioid-dependent patients, mixed agonist/antagonist analgesics may precipitate withdrawal symptoms.
Use in Ambulatory Patients
Levo-Dromoran has been used in both inpatient and outpatient settings, but both physicians and patients must be aware of the risk of orthostatic hypotension, dizziness and syncope in ambulatory patients.
As with other opioids, the use of Levo-Dromoran may impair mental and/or physical abilities required for the performance of potentially hazardous tasks or for the exercise of normal good judgement and patients and staff should be advised accordingly.
Concurrent use of Levo-Dromoran with central nervous system depressants (eg, alcohol, sedatives, hypnotics, other opioids, barbiturates, tricyclic antidepressants, phenothiazines, tranquilizers, skeletal muscle relaxants and antihistamines) may result in additive central nervous system depressant effects.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No information about the effects of Levo-Dromoran on carcinogenesis, mutagenesis, or fertility is available.
Pregnancy
Teratogenic Effects
Pregnancy Category C. Levo-Dromoran has been shown to be teratogenic in mice when given at a single oral dose of 25 mg/kg. The tested dose caused a near 50% mortality of the mouse embryos. There are no adequate and well-controlled studies in pregnant women. Levo-Dromoran should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Babies born to mothers who have been taking opioids regularly prior to delivery may be physically dependent.
A study in rabbits has demonstrated that at doses of 1.5 to 20 mg/kg, Levo-Dromoran administered intravenously crosses the placental barrier and depresses fetal respiration.
Labor and Delivery
The use of Levo-Dromoran in labor and delivery in humans has not been studied. However, as with other opioids, administration of Levo-Dromoran to the mother during labor and delivery may result in respiratory depression in the newborn. Therefore, its use during labor and delivery is not recommended.
Nursing Mothers
Studies of levorphanol concentrations in breast milk have not been performed. However, morphine, which is structurally similar to levorphanol, is excreted in human milk. Because of the potential for serious adverse reactions from Levo-Dromoran in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Levo-Dromoran is not recommended in children under the age of 18 years as the safety and efficacy of the drug in this population has not been established.
Geriatric Use
The initial dose of Levo-Dromoran should be reduced by 50% or more in the infirm elderly patient, even though there have been no reports of unexpected adverse events in older populations. All drugs of this class may be associated with a profound or prolonged effect in elderly patients for both pharmacokinetic and pharmacodynamic reasons and caution is indicated.
ADVERSE REACTIONS
In approximately 1400 patients treated with Levo-Dromoran in controlled clinical trials, the type and incidence of side effects were those expected of an opioid analgesic, and no unforeseen or unusual toxicity was reported.
Drugs of this type are expected to produce a cluster of typical opioid effects in addition to analgesia, consisting of nausea, vomiting, altered mood and mentation, pruritus, flushing, difficulties in urination, constipation and biliary spasm. The frequency and intensity of these effects appears to be dose related. Although listed as adverse events these are expected pharmacologic actions of these drugs and should be interpreted as such by the clinician.
The following adverse events have been reported with the use of Levo-Dromoran:
Body as a Whole: abdominal pain, dry mouth, sweating
Cardiovascular System: cardiac arrest, shock, hypotension, arrhythmias including bradycardia and tachycardia, palpitations, extra-systoles
Digestive System: nausea, vomiting, dyspepsia, biliary tract spasm
Nervous System: coma, suicide attempt, convulsions, depression, dizziness, confusion, lethargy, abnormal dreams, abnormal thinking, nervousness, drug withdrawal, hypokinesia, dyskinesia, hyperkinesia, CNS stimulation, personality disorder, amnesia, insomnia
Respiratory System: apnea, cyanosis, hypoventilation
Skin & Appendages: pruritus, urticaria, rash, injection site reaction
Special Senses: abnormal vision, pupillary disorder, diplopia
Urogenital System: kidney failure, urinary retention, difficulty urinating
DRUG ABUSE AND DEPENDENCE
Warning: May be Habit Forming
Levo-Dromoran is a Schedule II Controlled Substance. All drugs of this class (mu-opioids of the morphine type) are habit forming and should be stored, prescribed, used and disposed of accordingly. Psychological/physical dependence and tolerance may develop upon repeated administration of Levo-Dromoran.
Discontinuation of Levo-Dromoran after chronic use has been reported to result in withdrawal syndromes, and some reports of overuse and self-reported addiction have been received. Neither withdrawal nor withdrawal symptoms are usually expected in postoperative patients who used the drug for less than a week or in patients who are gradually tapered off the drug after longer use.
OVERDOSAGE
Most reports of overdosage known to the manufacturer and to the FDA involve three clinical situations. These are: 1. the use of larger than recommended doses or too frequent doses, 2. administration of the drug to children or small adults without any reduction in dosage, and 3. the use of the drug in ordinary dosage in patients compromised by concurrent illness.
As with all opioids, overdose can occur due to accidental or intentional misuse of this product, especially in infants and children who may gain access to the drug in the home. Based on its pharmacology, levorphanol overdosage would be expected to produce signs of respiratory depression, cardiovascular failure (especially in predisposed patients) and/or central nervous system depression. Serious overdosage with Levo-Dromoran is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, periodic breathing, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Treatment: The specific treatment of suspected levorphanol tartrate overdosage is immediate establishment of an adequate airway and ventilation, followed (if necessary) by intravenous naloxone. The respiratory and cardiac status of the patient should be continuously monitored and appropriate supportive measures instituted, such as oxygen, intravenous fluids and/or vasopressors, if required. Physicians are reminded that the duration of levorphanol action far exceeds the duration of action of naloxone, and repeated dosing with naloxone may be required. Naloxone should be administered cautiously to persons known or suspected to be physically dependent on Levo-Dromoran. In such cases an abrupt and complete reversal of opioid effects may precipitate an acute abstinence syndrome. If necessary to administer naloxone to the physically dependent patient, the antagonist should be administered with extreme care and by titration with smaller than usual doses of the antagonist.
DOSAGE AND ADMINISTRATION
Intravenous: The usual recommended starting dose for IV administration is up to 1 mg, given in divided doses, by slow injection. This may be repeated in 3 to 6 hours as needed, provided the patient is assessed for signs of hypoventilation or excessive sedation. Dosage should be adjusted according to the severity of the pain; age, weight and physical status of the patient; the patient’s underlying diseases; use of concomitant medications; and other factors (see INDIVIDUALIZATION OF DOSAGE, WARNINGS and PRECAUTIONS). Total daily doses of more than 4 to 8 mg IV in 24 hours are generally not recommended as starting doses in nonopioid tolerant patients; lower total daily doses may be appropriate.
Intramuscular or Subcutaneous: The usual recommended starting dose for IM or SC administration is 1 to 2 mg. This may be repeated in 6 to 8 hours as needed, provided the patient is assessed for signs of hypoventilation or excessive sedation. Dosage should be adjusted according to the severity of the pain; age, weight and physical status of the patient; the patient’s underlying diseases; use of concomitant medications; and other factors (see INDIVIDUALIZATION OF DOSAGE, WARNINGS and PRECAUTIONS). Total daily doses of more than 3 to 8 mg IM in 24 hours are generally not recommended as starting doses in nonopioid tolerant patients; lower total daily doses may be appropriate.
Oral: The usual recommended starting dose for oral administration is 2 mg. This may be repeated in 6 to 8 hours as needed, provided the patient is assessed for signs of hypoventilation and excessive sedation. If necessary, the dose may be increased to up to 3 mg every 6 to 8 hours, after adequate evaluation of the patient’s response. Higher doses may be appropriate in opioid tolerant patients. Dosage should be adjusted according to the severity of the pain; age, weight and physical status of the patient; the patient’s underlying diseases; use of concomitant medications; and other factors (see INDIVIDUALIZATION OF DOSAGE, WARNINGS and PRECAUTIONS). Total oral daily doses of more than 6 to 12 mg in 24 hours are generally not recommended as starting doses in nonopioid tolerant patients; lower total daily doses may be appropriate.
Use in Chronic Pain: The dosage of Levo-Dromoran in patients with cancer or with other conditions for which chronic opioid therapy is indicated must be individualized (see INDIVIDUALIZATION OF DOSAGE). Levo-Dromoran is 4 to 8 times as potent as morphine and has a longer half-life. Because there is incomplete cross-tolerance among opioids, when converting a patient from morphine to Levo-Dromoran, the total daily dose of oral Levo-Dromoran should begin at approximately 1/15 to 1/12 of the total daily dose of oral morphine that such patients had previously required and then the dose should be adjusted to the patient’s clinical response. If a patient is to be placed on fixed-schedule dosing (round-the-clock) with this drug, care should be taken to allow adequate time after each dose change (approximately 72 hours) for the patient to reach a new steady-state before a subsequent dose adjustment to avoid excessive sedation due to drug accumulation.
Use in The Perioperative Period: Levo-Dromoran has been used for analgesic action during premedication and the postoperative period. Factors to be considered in determining the dosage include age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia used, the surgical procedure involved and the severity of pain (see INDIVIDUALIZATION OF DOSAGE, WARNINGS and PRECAUTIONS).
Premedication: The preoperative medication dose of Levo-Dromoran should be individualized (see INDIVIDUALIZATION OF DOSAGE, WARNINGS and PRECAUTIONS). The usual dose for healthy young adults is 1 to 2 mg intramuscularly or subcutaneously, administered 60 to 90 minutes before surgery. Older or debilitated patients usually require less drug. Two mg of Levo-Dromoran is approximately equivalent to 10 to 15 mg of morphine or 100 mg of meperidine.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Pharmaceutical Incompatibilities of Levo-Dromoran: Levorphanol tartrate injection has been reported to be physically incompatible with solutions containing aminophylline, ammonium chloride, amobarbital sodium, chlorothiazide sodium, heparin sodium, methicillin sodium, nitrofurantoin sodium, novobiocin sodium, pentobarbital sodium, perphenazine, phenobarbital sodium, phenytoin sodium, secobarbital sodium, sodium bicarbonate, sodium iodide, sulfadiazine sodium, sulfisoxazole diethanolamine and thiopental sodium.
Safety and Handling: Levo-Dromoran is packaged in sealed systems that have a low risk of accidental exposure to health care workers. Ordinary care should be taken to avoid aerosol generation while preparing a syringe for use. Significant absorption from accidental dermal exposure is unlikely, and spilled Levo-Dromoran should be washed from the skin by rinsing with cool water. As with all controlled substances, abuse by health care personnel is possible and the drug should be handled accordingly.
HOW SUPPLIED
Ampuls: 1 mL, 2 mg/mL levorphanol tartrate – boxes of 10 (NDC 0187-3072-10).
Multiple-Dose Vials: 10 mL, 2 mg/mL levorphanol tartrate – boxes of 1 (NDC 0187-3074-20).
Scored Oral Tablets: 2 mg round, white, flat beveled edge tablets in bottles of 100 (NDC 0187-3251-10); with LEVO engraved on one side and 3251 and full bisect scored on the other side.
Storage: Tablets should be stored at 25°C (77°F); excursions permitted to 15°C- 30°C (59°F - 86°F).
Dispense in tight containers as defined in USP/NF.
Parenteral dosage forms should be stored at 25°C (77°F); excursions permitted to 15°C - 30°C (59°F - 86°F).
DEA Order Form Required.
Manufactured for:
Valeant Pharmaceuticals International
Costa Mesa, CA 92626
Valeant Pharmaceuticals International
3300 Hyland Ave., Costa Mesa, CA 92626 U.S.A.
714-545-0100
3325197EX03
Rev. April 2004
| LEVO-DROMORAN
levorphanol tartrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVO-DROMORAN
levorphanol tartrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVO-DROMORAN
levorphanol tartrate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Valeant Pharmaceuticals North America LLC (042230623) |