Label: OXYGEN gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 72223-002-01 - Packager: Praxair Mexico, S. de R.L. de C.V
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
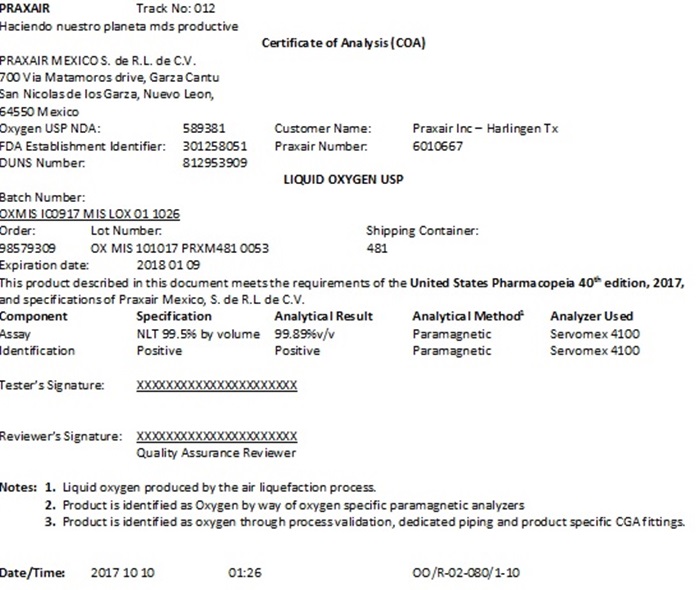
PRAXAIR Track No: 012
Haciendo nuestro planeta mds productive
Certificate of Analysis (COA)
PRAXAIR MEXICO S. de R.L. de C.V.
700 Via Matamoros drive, Garza Cantu
San Nicolas de los Garza, Nuevo Leon,
64550 Mexico
Oxygen USP NDA: 589381 Customer Name: Praxair Inc – Harlingen Tx
FDA Establishment Identifier: 301258051 Praxair Number: 6010667
DUNS Number: 812953909
LIQUID OXYGEN USP
Batch Number:
OXMIS IC0917 MIS LOX 01 1026
Order: Lot Number: Shipping Container:
98579309 OX MIS 101017 PRXM481 0053 481
Expiration date: 2018 01 09
This product described in this document meets the requirements of the United States Pharmacopeia 40th edition, 2017, and specifications of Praxair Mexico, S. de R.L. de C.V.
Component Specification Analytical ResultAnalytical Method1Analyzer Used
Assay NLT 99.5% by volume 99.89%v/v Paramagnetic Servomex 4100
Identification Positive Positive Paramagnetic Servomex 4100
Tester’s Signature: XXXXXXXXXXXXXXXXXXXXXX
Reviewer’s Signature: XXXXXXXXXXXXXXXXXXXXXX
Quality Assurance Reviewer
Notes:1. Liquid oxygen produced by the air liquefaction process.
2. Product is identified as Oxygen by way of oxygen specific paramagnetic analyzers
3. Product is identified as oxygen through process validation, dedicated piping and product specific CGA fittings.
Date/Time: 2017 10 10 01:26 OO/R-02-080/1-10
-
PRINCIPAL DISPLAY PANEL
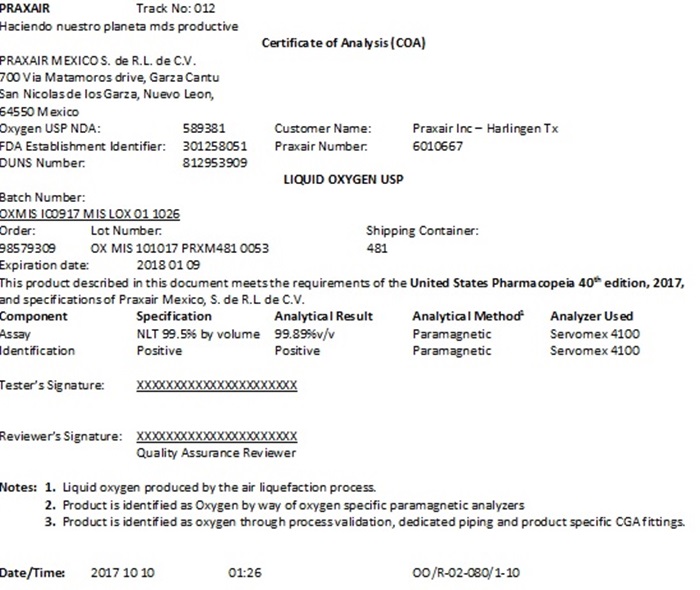
PRAXAIR Track No:
Haciendo nuestro planeta mds productive
Certificate of Analysis (COA)
PRAXAIR MEXICO S. de R.L. de C.V.
700 Via Matamoros drive, Garza Cantu
San Nicolas de los Garza, Nuevo Leon,
64550 Mexico
Oxygen USP NDA: 210844 Customer Name:
FDA Establishment Identifier: 3004658099 Praxair Number:
DUNS Number: 812821245
LIQUID OXYGEN USP
Batch Number: _________
Order: ________ Lot Number: __________ Shipping Container: ___________
Component Specification Analytical ResultAnalytical Method1Analyzer Used
Assay NLT 99.5% by volume _____%v/v Paramagnetic Servomex 4100
Identification Positive _________ Paramagnetic Servomex 4100
Tester’s Signature: __________________________
Reviewer’s Signature: _________________________
Quality Assurance Reviewer
Notes:1. Liquid oxygen produced by the air liquefaction process.
2. Product is identified as Oxygen by way of oxygen specific paramagnetic analyzers
3. Product is identified as oxygen through process validation, dedicated piping and product specific CGA fittings.
Date/Time: _______________________ OO/R-02-080/3-10

-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72223-002 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72223-002-01 12310000 L in 1 CONTAINER; Type 0: Not a Combination Product 06/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210844 06/08/2017 Labeler - Praxair Mexico, S. de R.L. de C.V (812953909) Establishment Name Address ID/FEI Business Operations Praxair Mexico, S. de R.L. de C.V 812953909 manufacture(72223-002) Establishment Name Address ID/FEI Business Operations Praxair Mexico, S. de R.L. de C.V 812821245 manufacture(72223-002)