ZOLPAK- econazole nitrate
PureTek Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Zolpak
DESCRIPTION
Econazole Nitrate Cream contains the antifungal agent, econazole nitrate 1% in a water miscible base consisting of pegoxol 7 stearate, peglicol 5 oleate, mineral oil, benzoic acid, butylated hydroxyanisole, and purified water. The white to off-white soft cream is for topical use only.
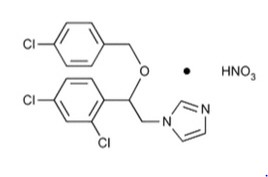
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl) methoxy}-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole mononitrate. Its structure is as follows:
CLINICAL PHARMACOLOGY
After topical application to the skin of normal subjects, systemic absorption of econazole nitrate is extremely low. Although most of the applied drug remains on the skin surface, drug concentrations were found in the stratum corneum which, by far, exceeded the minimum inhibitory concentration for dermatophytes. Inhibitory concentrations were achieved in the epidermis and as deep as the middle region of the dermis. Less than 1% of the applied dose was recovered in the urine and feces.
Microbiology
Econazole nitrate has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
| Dermatophytes | Yeasts |
| Epidermophyton floccosum | Candida albicans |
| Microsporum audouini | Malassezia furfur |
| Microsporum canis | |
| Microsporum gypseum | |
| Trichophyton mentagrophytes | |
| Trichophyton rubrum | |
| Trichophyton tonsurans |
Econazole nitrate exhibits broad-spectrum antifungal activity against the following organisms in vitro, but the clinical significance of these data is unknown.
|
Dermatophytes | Yeasts |
| Trichophyton verrucosum | Candida guillermondii |
| Candida parapsilosis | |
| Candida tropicalis |
INDICATIONS AND USAGE
Econazole Nitrate Cream is indicated for topical application in the treatment of tinea pedis, tinea cruris, and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Microsporum canis, Microsporum audouini, Microsporum gypseum, and Epidermophyton floccosum, in the treatment of cutaneous candidiasis, and in the treatment of tinea versicolor
CONTRAINDICATIONS
Econazole Nitrate Cream is contraindicated in individuals who have shown hypersensitivity to any of its ingredients.
Carcinogenicity Studies
Long-term animal studies to determine carcinogenic potential have not been performed.
Oral administration of econazole nitrate in rats has been reported to produce prolonged gestation. Intravaginal administration in humans has not shown prolonged gestation or other adverse reproductive effects attributable to econazole nitrate therapy.
Fertility (Reproduction)
Oral administration of econazole nitrate in rats has been reported to produce prolonged gestation. Intravaginal administration in humans has not shown prolonged gestation or other adverse reproductive effects attributable to econazole nitrate therapy.
Pregnancy
Econazole nitrate has not been shown to be teratogenic when administered orally to mice, rabbits or rats. Fetotoxic or embryotoxic effects were observed in Segment I oral studies with rats receiving 10 to 40 time the human dermal dose. Similar effects were observed in Segment II or Segment III studies with mice, rabbits and/or rats receiving oral doses 80 or 40 time the human dermal dose.
Econazole nitrate should be used in the first trimester of pregnancy only when the physician considers it essential to the welfare of the patient. The drug should be used during the second and third trimesters of pregnancy only if clearly needed.
Nursing Mothers
It is not known whether econazole nitrate is excreted in human milk. Following oral administration of econazole nitrate to lactating rats, econazole and/or metabolites were excreted in milk and were found in nursing pups. Also, in lactating rats receiving large oral doses (40 or 80 times the human dermal dose), there was a reduction in post partum viability of pups and survival to weaning; however, at these high doses, maternal toxicity was present and may have been a contributing factor. Caution should be exercised when econazole nitrate is administered to a nursing woman.
ADVERSE REACTIONS
During clinical trials, approximately 3% of patients treated with econazole nitrate 1% cream reported side effects thought possibly to be due to the drug, consisting mainly of burning, itching, stinging, and erythema. One case of pruritic rash has also been reported.
To report suspected adverse reactions, contact Teligent Pharma, Inc. at 1-856-697-1441, or FDA at 1-800-FDA-1088 or 1-800-332-1088 or www.fda.gov/medwatch.
OVERDOSE
Overdosage of econazole nitrate in humans has not been reported to date. In mice, rats, guinea pigs and dogs, the oral LD 50 values were found to be 462, 668, 272, and >160 mg/kg, respectively.
DOSAGE AND ADMINISTRATION
Sufficient Econazole Nitrate Cream 1% should be applied to cover affected areas once daily in patients with tinea pedis, tinea cruris, tinea corporis, and tinea versicolor, and twice daily (morning and evening) in patients with cutaneous candidiasis.
Early relief of symptoms is experienced by the majority of patients and clinical improvement may be seen fairly soon after treatment is begun; however, candidal infections and tinea cruris and corporis should be treated for two weeks and tinea pedis for one month in order to reduce the possibility of recurrence. If a patient shows no clinical improvement after the treatment period, the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
HOW SUPPLIED
Econazole Nitrate Cream 1% is supplied in the following:
15 gram tubes (NDC 52565-022-15)
30 gram tubes (NDC 52565-022-30)
85 gram tubes (NDC 52565-022-85)
FRAME STYLE TRANSPARENT DRESSING
INSTRUCTIONS FOR USE
DESCRIPTIONS
Frame Style Transparent Dressing are designed to allow oxygen and moisture vapor exchange, yet provide a barrier to resist liquids and bacteria. They are comfortable, and flex with skin for patient comfort.
INDICATIONS FOR USE
Frame Style Transparent Dressing are indicated for I.V. sites, surgical incise, primary dressing on pressure ulcers (stages I and II) with minimal drainage, partial thickness wounds, and skin tears or abrasions. They are also used to aid in autolylic debridement.
CONTRAINDICATIONS
Frame Style Transparent Dressing are contraindicated as a primary dressing on moderately to heavily draining wounds. Avoid repeated applications on patients with thin or fragile skin that may result in skin damage.
APPLICATION
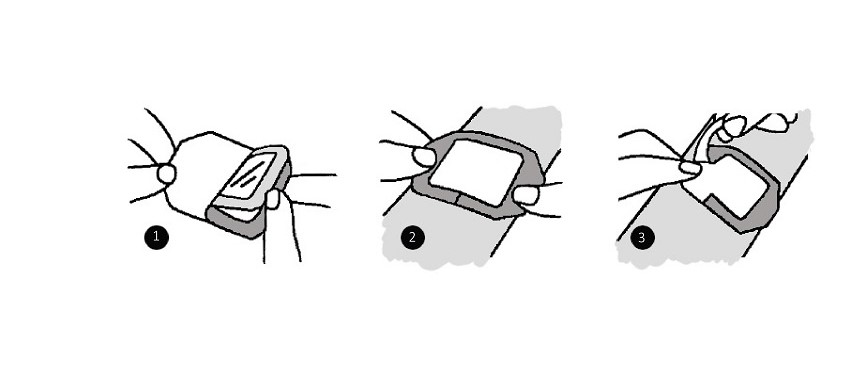
1. Prepare the wound or site according to facility protocol. Allow all skin cleansing and preparation solutions to dry completely.
2. Remove dressing from package. Peel back lining from the dressing.
3. position the dressing ove the wound or site and apply using light pressure. Do not stretch the dressing while applying.
4. Gently remove the frame, smoothing the dressing down as the frame is being pulled away.
5. Smooth the dressing from the center toward the edges to aid adhesion.
6. Date and initial the dressing in the spaces provided.
REMOVAL
1. Change dressing according to standard wound care practices and facility protocol.
2. Supporting the skin, gently lift corner and slowly peel the dressing from the skin in the direction of the hair growth. Peel the dressing back, parallel to the skin, rather than pulling it up from the skin.
3. Dispose of the dressing according to facility protocol.
PRECAUTIONS FOR USE
1. To assure good adhesion, the skin should be clean, dry and free from skin oils, soaps, detergents and lotions. Ensure that no wet skin preparationsolution or soap residues are trapped under the dressing.
2. The dressing should be allowed a minimum overlap of 1 inch (3 cm) from the margin of the wound onto the surrounding healthy skin.
3. Removed excess hair from around the site, as required, by clipping not shaving. Do not reuse.
STORAGE: Avoid storage in direct sunlight/flourescent lighting and keep area cool, dry, and well-ventilated.
Contents STERILE in unopened, undamaged inner package. Not made with natural rubber latex.
| ZOLPAK
econazole nitrate kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - PureTek Corporation (785961046) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Teligent Pharma, Inc. | 011036910 | manufacture(52565-022) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PureTek Corporation | 785961046 | pack(59088-752) | |