AMINOHIPPURATE SODIUM PAH- aminohippurate sodium injection, solution
Merck Sharp & Dohme Corp.
----------
AMINOHIPPURATE SODIUM "PAH"
INJECTION
DESCRIPTION
Aminohippurate sodium1 is an agent to measure effective renal plasma flow (ERPF). It is the sodium salt of para-aminohippuric acid, commonly abbreviated "PAH". It is water soluble, lipid-insoluble, and has a pKa of 3.83. The empirical formula of the anhydrous salt is C9H9N2NaO3 and its structural formula is:
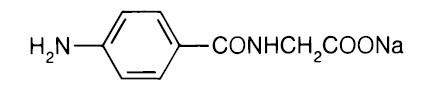
It is provided as a sterile, non-preserved 20 percent aqueous solution for injection, with a pH of 6.7 to 7.6. Each 10 mL contains: Aminohippurate sodium 2 g. Inactive ingredients: Sodium hydroxide to adjust pH, water for injection, q.s.
- 1
-
Formerly referred to as Sodium para-Aminohippurate.
CLINICAL PHARMACOLOGY
PAH is filtered by the glomeruli and is actively secreted by the proximal tubules. At low plasma concentrations (1.0 to 2.0 mg/100 mL), an average of 90 percent of PAH is cleared by the kidneys from the renal blood stream in a single circulation. It is ideally suited for measurement of ERPF since it has a high clearance, is essentially nontoxic at the plasma concentrations reached with recommended doses, and its analytical determination is relatively simple and accurate.
PAH is also used to measure the functional capacity of the renal tubular secretory mechanism or transport maximum (TmPAH). This is accomplished by elevating the plasma concentration to levels (40-60 mg/100 mL) sufficient to saturate the maximal capacity of the tubular cells to secrete PAH.
Inulin clearance is generally measured during TmPAH determinations since glomerular filtration rate (GFR) must be known before calculations of secretory Tm measurements can be done (see DOSAGE AND ADMINISTRATION, Calculations).
INDICATIONS AND USAGE
Estimation of effective renal plasma flow.
Measurement of the functional capacity of the renal tubular secretory mechanism.
PRECAUTIONS
General
Intravenous solutions must be given with caution to patients with low cardiac reserve, since a rapid increase in plasma volume can precipitate congestive heart failure.
For measurement of ERPF, small doses of PAH are used. However, in research procedures to measure TmPAH, high plasma levels are required to saturate the capacity of the tubular cells. During these procedures, the intravenous administration of PAH solutions should be carried out slowly and with caution. The patient should be continuously observed for any adverse reactions.
Use caution when injecting this product into latex-sensitive individuals, since the vial stopper contains dry natural latex rubber that may cause allergic reactions.
Drug Interactions
Renal clearance measurements of PAH cannot be made with any significant accuracy in patients receiving sulfonamides, procaine, or thiazolesulfone. These compounds interfere with chemical color development essential to the analytical procedures.
Probenecid depresses tubular secretion of certain weak acids such as PAH. Therefore, patients receiving probenecid will have erroneously low ERPF and TmPAH values.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been done to evaluate any effects upon fertility or carcinogenic potential of PAH.
ADVERSE REACTIONS
Hypersensitivity reactions including anaphylaxis, angioedema, urticaria, vasomotor disturbances, flushing, tingling, nausea, vomiting, and cramps may occur.
Patients may have a sensation of warmth or the desire to defecate or urinate during or shortly following initiation of infusion.
DOSAGE AND ADMINISTRATION
For intravenous use only
Clearance measurements using single injection techniques are generally inaccurate, particularly in the measurement of ERPF. For this reason, intravenous infusions at fixed rates are used to sustain the plasma PAH concentration at the desired level.
To measure ERPF, the concentration of PAH in the plasma should be maintained at 2 mg per 100 mL, which can be achieved with a priming dose of 6 to 10 mg/kg and an infusion dose of 10 to 24 mg/min.
As a research procedure for the measurement of TmPAH, the plasma level of PAH must be sufficient to saturate the capacity of the tubular secretory cells. Concentrations from 40 to 60 mg per 100 mL are usually necessary.
Technical details of these tests may be found in Smith {1}; Wesson {2}; Bauer {3}; Pitts{4}; and Schnurr {5}.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to use, whenever solution and container permit. NOTE: The normal color range for this product is a colorless to yellow/brown solution. The efficacy is not affected by color changes within this range.
Calculations
Effective Renal Plasma Flow (ERPF)
The clearance of PAH, which is extracted almost completely from the plasma during its passage through the renal circulation, constitutes a measure of ERPF. Hence:
ERPF = UPAHV/PPAH
Where UPAH = concentration of PAH (mg/mL) in the urine
V = rate of urine excretion (mL/min), and
PPAH = plasma concentration of PAH (mg/mL).
Example:
UPAH = 8.0 mg/mL
V = 1.5 mL/min
PPAH = 0.02 mg/mL
ERPF = 8.0 x 1.5/0.02 = 600 mL/min
Based on PAH clearance studies, the normal values for ERPF are:
men 675 ± 150 mL/min
women 595 ± 125 mL/min
Maximum Tubular Secretory
(TmPAH ) Mechanism
The quantity of PAH secreted by the tubules (TmPAH) is given by the difference between the total rate of excretion (UPAHV) and the quantity filtered by the glomeruli (GFR x PPAH). Hence:
TmPAH = UPAHV – (GFR x PPAH x 0.83)
The factor, 0.83, corrects for that portion of PAH which is bound to plasma protein and hence is unfilterable.
Example:
UPAH = 9.55 mg/mL
V = 16.68 mL/min
GFR = 120 mL/min
PPAH = 0.60 mg/mL
Then TmPAH = 9.55 x 16.68 – (120 x 0.60 x 0.83) = 100 mg/min.
Average normal values of TmPAH are 80-90 mg/min.
The value of the expression UPAHV, used in calculations of ERPF and TmPAH, may be found by determining the amount of PAH in a measured volume of urine excreted within a specific period of time.
These calculations are based on a body surface area of 1.73 m2. Corrections for variations in surface area are made by multiplying the values obtained for ERPF and TmPAH by 1.73/A, where A is the subject surface area.
HOW SUPPLIED
No. 95 — Aminohippurate Sodium, 20 percent sterile solution for intravenous injection, is supplied as follows:
NDC 0006-3395-11 in 10 mL vials.
REFERENCES
- Smith, H.W.: Lectures on the kidney, University Extension Division, University of Kansas, Lawrence, Kansas, 1943.
- Wesson, L.G., Jr.: "Physiology of the Human Kidney," New York, Grune & Stratton, 1969, pp. 632-655.
- Bauer, J.D.; Ackermann, P.G.; Toro, G.: "Brays Clinical Laboratory Methods," ed. 7, St. Louis, Mosby, 1968.
- Pitts, R.F.: "Physiology of the Kidney and Body Fluids," ed. 2, Chicago, Year Book Medical Publishers, 1968.
- Schnurr, E.; Lahme, W.; Kuppers, H.: Measurement of renal clearance of inulin and PAH in the steady state without urine collection; Clinical Nephrology, 13(1): (26-29), 1980.
Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Issued January 2011
Printed in USA
9051026
Copyright © 1983 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved
This is a representative sample of the packaging. Please see How Supplied section for a complete list of available packaging.
PRINCIPAL DISPLAY PANEL - Carton - 10 mL Single Dose Vial
NDC 0006-3395-11
SINGLE DOSE VIAL
10 mL INJECTION
AMINOHIPPURATE Sodium
2 g in 10 mL
(20% solution)
FOR INTRAVENOUS USE ONLY TO DETERMINE KIDNEY FUNCTION
Rx only
Merck Sharp & Dohme Corp.,
a subsidiary of
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA

| AMINOHIPPURATE SODIUM
PAH
aminohippurate sodium injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme Corp. (001317601) |