OMNARIS- ciclesonide spray
Sunovion Pharmaceuticals Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OMNARIS® safely and effectively. See full prescribing information for OMNARIS.
OMNARIS® (ciclesonide) nasal spray Initial U.S. Approval: 2006 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONFor Intranasal Use Only DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (>2% incidence) included headache, epistaxis, nasopharyngitis, ear pain, and pharyngolaryngeal pain. (6) To report SUSPECTED ADVERSE REACTIONS, contact Sunovion Pharmaceuticals Inc. at 1-877-737-7226 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch . See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 3/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
Administer OMNARIS Nasal Spray by the intranasal route only. Prior to initial use, OMNARIS Nasal Spray must be gently shaken and then the pump must be primed by actuating eight times. If the product is not used for four consecutive days, it should be gently shaken and reprimed with one spray or until a fine mist appears. Illustrated patient's instructions for proper use accompany each package of OMNARIS Nasal Spray.
3 DOSAGE FORMS AND STRENGTHS
OMNARIS Nasal Spray is a metered-dose, manual-pump spray formulation containing a hypotonic aqueous suspension of ciclesonide. Once primed, each actuation of the pump delivers 50 mcg ciclesonide in a volume of 70 microliters from the nasal actuator.
4 CONTRAINDICATIONS
OMNARIS Nasal Spray is contraindicated in patients with a known hypersensitivity to ciclesonide or any of the ingredients of OMNARIS Nasal Spray [see Warnings and Precautions (5.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Local Nasal Effects
Epistaxis: In clinical studies of 2 to 52 weeks' duration, epistaxis was observed more frequently in patients treated with OMNARIS Nasal Spray than those who received placebo [see Adverse Reactions (6)].
Candida Infection: In clinical studies with OMNARIS Nasal Spray, the development of localized infections of the nose and pharynx with Candida albicans has occurred. When such an infection develops, it may require treatment with appropriate local therapy and discontinuation of OMNARIS Nasal Spray. Therefore, patients using OMNARIS Nasal Spray over several months or longer should be examined periodically for evidence of Candida infection or other signs of adverse effects on the nasal mucosa.
Nasal Septal Perforation: Instances of nasal septal perforation have been reported in patients following the intranasal application of corticosteroids. No cases of nasal septal perforation were identified in clinical studies with OMNARIS Nasal Spray. Avoid spraying OMNARIS Nasal Spray directly onto the nasal septum.
5.2 Glaucoma and Cataracts
Nasal and inhaled corticosteroids may result in the development of glaucoma and/or cataracts. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
The risk of glaucoma was evaluated by assessments of intraocular pressure in 3 studies including 943 patients. Of these, 390 adolescents or adults were treated for up to 52 weeks and 186 children ages 2 to 11 received treatment with OMNARIS Nasal Spray 200 mcg daily for up to 12 weeks. In these studies, no significant differences in intraocular pressure changes were observed between OMNARIS Nasal Spray 200 mcg and placebo-treated patients. Additionally, no significant differences between OMNARIS Nasal Spray 200 mcg and placebo-treated patients were noted during the 52-week study of adults and adolescent patients in whom thorough ophthalmologic assessments were performed, including evaluation of cataract formation using slit lamp examinations.
5.3 Immunosuppression
Patients who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; or in patients with untreated local or systemic fungal or bacterial infections; systemic viral or parasitic infections; or ocular herpes simplex because of the potential for worsening of these infections.
5.4 Hypothalamic-Pituitary-Adrenal Axis Effect
Hypercorticism and Adrenal Suppression: When intranasal corticosteroids are used at higher than recommended dosages or in susceptible individuals at recommended dosages, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. If such changes occur, the dosage of OMNARIS Nasal Spray should be discontinued slowly, consistent with accepted procedures for discontinuing oral steroid therapy.
The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency. In addition, some patients may experience symptoms of corticosteroid withdrawal, e.g., joint and/or muscular pain, lassitude, and depression. Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress. In those patients who have asthma or other clinical conditions requiring long-term systemic corticosteroid treatment, rapid decreases in systemic corticosteroid dosages may cause a severe exacerbation of their symptoms.
6 ADVERSE REACTIONS
Systemic and local corticosteroid use may result in the following:
- Epistaxis, nasal septal perforations, Candida albicans infection, impaired wound healing [see Warnings and Precautions (5.1)]
- Cataracts and glaucoma [see Warnings and Precautions (5.2)]
- Immunosuppression [see Warnings and Precautions (5.3)]
- Hypothalamic-pituitary-adrenal (HPA) axis effects, including growth reduction [see Warnings and Precautions (5.4, 5.5), Use in Specific Populations (8.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below for adults and adolescents 12 years of age and older are based on 3 clinical trials of 2 to 6 weeks duration and one 52-week trial. In the 3 trials of 2 to 6 weeks duration, 1524 patients (495 males and 1029 females, ages 12 to 86 years old) with seasonal or perennial allergic rhinitis were treated with OMNARIS Nasal Spray 200, 100, 50, or 25 mcg or placebo once daily. The racial distribution in these three trials included 1374 Caucasians, 69 Blacks, 31 Asians, and 50 patients classified as Other. The 52-week trial was conducted in 663 patients (227 males and 436 females, ages 12 to 73 years old) treated with OMNARIS Nasal Spray 200 mcg or placebo once daily. The racial distribution in this trial included 538 Caucasians, 69 Blacks, 16 Asians, and 40 patients classified as Other. The data from pediatric patients are based upon 4 clinical trials in which 1541 children (871 males and 670 females, ages 2 to 11 years old) with seasonal or perennial allergic rhinitis were treated with OMNARIS Nasal Spray 200, 100, or 25 mcg or placebo once daily for 2 to 12 weeks. The racial distribution in these four trials included 1136 Caucasians, 273 Blacks, 20 Asians, and 112 patients classified as Other.
Adults and Adolescents 12 Years of Age and Older in Short-Term (2-6 weeks) Trials: In three short-term trials conducted in the US and Canada, 546 patients were treated with OMNARIS Nasal Spray 200 mcg daily. Adverse reactions did not differ appreciably based on age, gender, or race. Approximately 2% of patients treated with OMNARIS Nasal Spray 200 mcg in clinical trials discontinued because of adverse reactions; this rate was similar for patients treated with placebo. The table below displays reactions that occurred with an incidence of 2% or greater and more frequently with OMNARIS Nasal Spray 200 mcg than with placebo in clinical trials of 2 to 6 weeks in duration.
| Adverse Event | OMNARIS Nasal Spray 200 mcg Once Daily (N = 546) % | Placebo (N = 544) % |
| Headache | 6.0 | 4.6 |
| Epistaxis | 4.9 | 2.9 |
| Nasopharyngitis | 3.7 | 3.3 |
| Ear Pain | 2.2 | 0.6 |
Pediatric Patients Aged 6 to 11 Years in Short-Term (2-12 weeks) Trials: In two short-term trials, conducted in the US and Canada, 913 patients were treated with OMNARIS Nasal Spray 200 mcg, 100 mcg or 25 mcg daily. Adverse events did not differ appreciably based on age, gender, or race. In clinical trials, 1.6% and 2.7% of patients treated with OMNARIS Nasal Spray 200 mcg or 100 mcg, respectively, discontinued because of adverse reactions; these rates were lower than the rate in patients treated with placebo (2.8%). Table 2 displays adverse events that occurred with an incidence of 3% or greater and more frequently with OMNARIS Nasal Spray 200 mcg than with placebo.
| Adverse Event | OMNARIS Nasal Spray 200 mcg Once Daily (N = 380) % | Placebo (N = 369) % |
| Headache | 6.6 | 5.7 |
| Nasopharyngitis | 6.6 | 5.4 |
| Pharyngolaryngeal pain | 3.4 | 3.3 |
Pediatric Patients Aged 2 to 5 Years in Short-Term (6-12 weeks) Trials: In two short-term trials conducted in the US, 183 patients were treated with OMNARIS Nasal Spray 200 mcg, 100 mcg or 25 mcg daily. The distribution of adverse events was similar to that seen in the 6 to 11 year old children.
Long-Term (52-Week) Safety Trial: In a 52-week double-blind, placebo-controlled safety trial that included 663 adults and adolescent patients (441 treated with ciclesonide: 227 males and 436 females) with perennial allergic rhinitis, the adverse reaction profile over the treatment period was similar to the adverse event profile in trials of shorter duration. Adverse reactions, irrespective of drug relationship, that occurred with an incidence of 3% or greater and more frequently with OMNARIS Nasal Spray 200 mcg than with placebo were epistaxis, pharyngolaryngeal pain, sinusitis, headache, nasal discomfort, cough, bronchitis, influenza, back pain, and urinary tract infection. No patient experienced a nasal septal perforation or nasal ulcer during this long-term trial of OMNARIS Nasal Spray.
6.2 Post-Marketing Experience
The following adverse reactions have been reported in association with post-marketing use of OMNARIS Nasal Spray and are not listed above: nasal congestion, nasal ulcer and dizziness. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
In vitro studies and clinical pharmacology studies suggested that des-ciclesonide has no potential for metabolic drug interactions or protein binding-based drug interactions [see Clinical Pharmacology (12.3)].
In a drug interaction study, co-administration of orally inhaled ciclesonide and oral ketoconazole, a potent inhibitor of cytochrome P450 3A4, increased the exposure (AUC) of des-ciclesonide by approximately 3.6-fold at steady state, while levels of ciclesonide remained unchanged. Erythromycin, a moderate inhibitor of cytochrome P450 3A4, had no effect on the pharmacokinetics of either des-ciclesonide or erythromycin following oral inhalation of ciclesonide [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. OMNARIS Nasal Spray should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Oral administration of ciclesonide in rats at approximately 35 times the maximum human daily intranasal dose in adults based on mcg/m2 produced no teratogenicity or other fetal effects. However, subcutaneous administration of ciclesonide in rabbits at less than the maximum human daily intranasal dose in adults based on mcg/m2 produced fetal toxicity. This included fetal loss, reduced fetal weight, cleft palate, skeletal abnormalities including incomplete ossifications, and skin effects [see Nonclinical Toxicology (13.2)].
8.3 Nursing Mothers
It is not known if ciclesonide is excreted in human milk. However, other corticosteroids are excreted in human milk. In a study with lactating rats, minimal but detectable levels of ciclesonide were recovered in milk. Caution should be used when OMNARIS Nasal Spray is administered to nursing women.
8.4 Pediatric Use
The safety and effectiveness for seasonal and perennial allergic rhinitis in children 12 years of age and older have been established. The efficacy of OMNARIS Nasal Spray in patients 6 to 11 years of age for treatment of the symptoms of seasonal allergic rhinitis was demonstrated in one study in patients 6 to 11 years of age with seasonal allergic rhinitis. The efficacy of OMNARIS Nasal Spray for the treatment of the symptoms of seasonal allergic rhinitis in patients 5 years of age and younger has not been established. The efficacy of OMNARIS Nasal Spray for the treatment of the symptoms of perennial allergic rhinitis in patients 11 years of age and younger has not been established [see Clinical Studies (14.1)]. The safety of OMNARIS Nasal Spray in children 2 to 11 years of age was evaluated in 4 controlled clinical studies of 2 to 12 weeks duration [see Clinical Pharmacology (12.2), Clinical Studies (14.1), and Adverse Reactions (6)].
Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA)-axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA-axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including OMNARIS Nasal Spray, should be monitored routinely (e.g., via stadiometry). A 52-week, multicenter, double-blind, randomized, placebo-controlled parallel-group study was conducted to assess the effect of orally inhaled ciclesonide on growth rate in 609 pediatric patients with mild persistent asthma, aged 5 to 8.5 years. Treatment groups included orally inhaled ciclesonide 40 mcg or 160 mcg or placebo given once daily. Growth was measured by stadiometer height during the baseline, treatment and follow-up periods. The primary comparison was the difference in growth rates between ciclesonide 40 and 160 mcg and placebo groups. Conclusions cannot be drawn from this study because compliance could not be assured. Ciclesonide blood levels were also not measured during the one-year treatment period. There was no difference in efficacy measures between the placebo and the orally inhaled ciclesonide groups.
The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of safe and effective noncorticosteroid treatment alternatives. To minimize the systemic effects of intranasal corticosteroids, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
8.5 Geriatric Use
Clinical studies of OMNARIS Nasal Spray did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
Chronic overdosage may result in signs or symptoms of hypercorticism [see Warnings and Precautions (5.4)].
There are no data available on the effects of acute or chronic overdosage with OMNARIS Nasal Spray.
11 DESCRIPTION
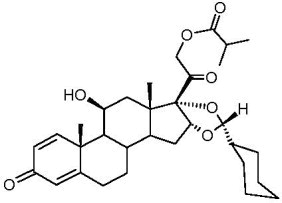
The active component of OMNARIS Nasal Spray is ciclesonide, a non-halogenated glucocorticoid having the chemical name pregna -1,4-diene-3,20-dione, 16,17-[[R-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21-(2-methyl-1-oxopropoxy)-,(11β,16α)-. Ciclesonide is delivered as the R-epimer. The empirical formula is C32H44O7 and its molecular weight is 540.7. Its structural formula is as follows:
Ciclesonide is a white to yellow-white powder, practically insoluble in water and freely soluble in ethanol and acetone. OMNARIS Nasal Spray is a metered-dose, manual-pump spray formulation containing a hypotonic aqueous suspension of ciclesonide. OMNARIS Nasal Spray also contains microcrystalline cellulose, carboxymethylcellulose sodium, hypromellose, potassium sorbate and edetate sodium; and hydrochloric acid to adjust the pH to 4.5.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ciclesonide is a pro-drug that is enzymatically hydrolyzed to a pharmacologically active metabolite, C21-desisobutyryl-ciclesonide (des-ciclesonide or RM1) following intranasal application. Des-ciclesonide has anti-inflammatory activity with affinity for the glucocorticoid receptor that is 120 times higher than the parent compound.
The precise mechanism through which ciclesonide affects allergic rhinitis symptoms is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic inflammation.
12.2 Pharmacodynamics
Adrenal Function: In a 6-week trial in adolescents and adults 12-73 years of age with perennial allergic rhinitis, a daily dose of 200 mcg of OMNARIS Nasal Spray was compared to placebo nasal spray. Dexamethasone 6 mg was used as an active control during the last 4 days of the treatment period. Adrenal function was assessed by measurement of 24 hour serum cortisol levels before and after 6 consecutive weeks of treatment. The difference from placebo for the change from baseline in serum cortisol AUC(0-24) was 10.4 mcg•hour/dL (95% CI: -4.7, 25.5) for 200 mcg of OMNARIS Nasal Spray. The effects observed with the active control (dexamethasone, n=18) validate the sensitivity of the study to assess the effect of ciclesonide on the HPA axis.
In a 12-week study in children 6 to 11 years of age with perennial allergic rhinitis, daily doses of 200 mcg, 100 mcg, and 25 mcg of OMNARIS Nasal Spray were compared to placebo nasal spray. Adrenal function was assessed by measurement of 24-hour urinary-free cortisol (in 32 to 44 patients per group) and morning plasma cortisol levels (in 45 to 61 patients per group) before and after 12 consecutive weeks of treatment. The ciclesonide-treated groups had a numerically greater decline in 24-hour urinary-free cortisol compared to the placebo-treated group. The differences (and 95% confidence intervals) from placebo in the mean change from baseline to 12 weeks were -0.81 (-4.0, 2.4), -0.08 (-3.1, 2.9), and -2.11 (-5.3, 1.1) mcg/day for 200 mcg, 100 mcg, and 25 mcg dose groups, respectively. The mean AM plasma cortisol value did not show any consistent treatment effect with differences (and 95% confidence intervals) from placebo in the mean change from baseline to 12 weeks of 0.35 (-1.4, 2.1), 0.12 (-1.5, 1.7), and -0.38 (-2.1, 1.3) mcg/dL for 200 mcg, 100 mcg, and 25 mcg dose groups, respectively. In this study, serum was assayed for ciclesonide and des-ciclesonide [see Clinical Pharmacology (12.3)].
In a 6-week study in children 2 to 5 years of age with perennial allergic rhinitis, daily doses of 200 mcg, 100 mcg, and 25 mcg of OMNARIS Nasal Spray were compared to placebo nasal spray. Adrenal function was assessed by measurement of 24-hour urinary-free cortisol (in 15 to 22 patients per group) and morning plasma cortisol levels (in 28 to 30 patients per group) before and after 6 consecutive weeks of treatment. The ciclesonide-treated groups had a numerically greater decline in 24-hour urinary-free cortisol compared to the placebo-treated group. The differences (and 95% confidence intervals) from placebo in the mean change from baseline to 6 weeks were -2.04 (-4.4, 0.3), -1.96 (-4.5, 0.6), and -1.76 (-4.3, 0.8) mcg/day for the 200 mcg, 100 mcg, and 25 mcg dose groups, respectively. The plasma cortisol also decreased numerically after treatment with ciclesonide. The differences (and 95% confidence intervals) from placebo in the mean change in plasma cortisol from baseline to 6 weeks were -1.04 (-2.7, 0.7), -0.36 (-2.1, 1.4), and -0.12 (-1.8, 1.6) mcg/dL for the 200 mcg, 100 mcg, and 25 mcg dose groups, respectively. In this study, serum was assayed for ciclesonide and des-ciclesonide [see Clinical Pharmacology (12.3)].
12.3 Pharmacokinetics
Absorption: Ciclesonide and des-ciclesonide have negligible oral bioavailability (both less than 1%) due to low gastrointestinal absorption and high first-pass metabolism. The intranasal administration of ciclesonide at recommended doses results in negligible serum concentrations of ciclesonide. However, the known active metabolite (des-ciclesonide) is detected in the serum of some patients after nasal inhalation of ciclesonide. The bioanalytical assay used has a lower limit of quantification of 25 pg/mL and 10 pg/mL, for ciclesonide and des-ciclesonide, respectively.
In healthy adults treated for two weeks with 50 to 800 mcg of ciclesonide nasal spray daily (n=6 in each treatment group), the peak serum concentrations of des-ciclesonide in all subjects were found to be below 30 pg/mL. Of those treated with 800 mcg and 400 mcg daily, 100% and 67% had detectable levels of des-ciclesonide, respectively. With daily doses of 200 mcg or less, detectable serum levels of des-ciclesonide were not observed. The low systemic exposure following ciclesonide nasal spray administration was confirmed in a crossover study in twenty-nine healthy adults. The median Cmax was less than 10 pg/mL and 602 pg/mL following a single dose of ciclesonide nasal spray (300 mcg) and orally inhaled ciclesonide (320 mcg), respectively.
Distribution: Following intravenous administration of 800 mcg of ciclesonide, the volumes of distribution of ciclesonide and des-ciclesonide were approximately 2.9 L/kg and 12.1 L/kg, respectively. The percentage of ciclesonide and des-ciclesonide bound to human plasma proteins averaged ≥ 99% each, with ≤ 1% of unbound drug detected in the systemic circulation. Des-ciclesonide is not significantly bound to human transcortin.
Metabolism: Ciclesonide is hydrolyzed to a biologically active metabolite, des-ciclesonide, by esterases. Des-ciclesonide undergoes further metabolism in the liver to additional metabolites mainly by the cytochrome P450 (CYP) 3A4 isozyme and to a lesser extent by CYP 2D6. The full range of potentially active metabolites of ciclesonide has not been characterized. After intravenous administration of 14C-ciclesonide, 19.3% of the resulting radioactivity in the plasma is accounted for by ciclesonide or des-ciclesonide; the remainder may be a result of other, as yet, unidentified multiple metabolites.
Elimination: Following intravenous administration of 800 mcg of ciclesonide, the clearance values of ciclesonide and des-ciclesonide were high (approximately 152 L/h and 228 L/h, respectively). 14C-labeled ciclesonide was predominantly excreted via the feces after intravenous administration (66%) indicating that excretion through bile is the major route of elimination. Approximately 20% or less of drug-related radioactivity was excreted in the urine.
Special Populations: The pharmacokinetics of intranasally administered ciclesonide have not been assessed in patient subpopulations because the resulting blood levels of ciclesonide and des-ciclesonide are insufficient for pharmacokinetic calculations. However, population pharmacokinetic analysis showed that characteristics of des-ciclesonide after oral inhalation of ciclesonide were not appreciably influenced by a variety of subject characteristics such as body weight, age, race, and gender.
Hepatic Impairment: Compared to healthy subjects, the systemic exposure (Cmax and AUC) in patients with liver impairment increased in the range of 1.4 to 2.7-fold after ex-actuator administration of 1280 mcg ciclesonide via oral inhalation. Dose adjustment in liver impairment is not necessary.
Renal Impairment: Studies in renally-impaired patients were not conducted since renal excretion of des-ciclesonide is a minor route of elimination (≤ 20%).
Pediatric: In pediatric subjects treated with 25 to 200 mcg of ciclesonide nasal spray daily, serum concentrations of des-ciclesonide were below 45 pg/mL, with the exception of one value of 64.5 pg/mL. In a 12-week study in children 6 to 11 years of age with perennial allergic rhinitis, des-ciclesonide was detected in 50% of the subjects treated with 200 mcg and in 5% of those treated with 100 mcg ciclesonide nasal spray daily. In a 6-week study in children 2 to 5 years of age with perennial allergic rhinitis, des-ciclesonide was detected in 41%, 22%, and 13% of the subjects treated with 200 mcg, 100 mcg, and 25 mcg ciclesonide nasal spray daily, respectively.
Drug-Drug Interactions: Based on in vitro studies in human liver microsomes, des-ciclesonide appears to have no inhibitory or induction potential on the metabolism of other drugs metabolized by cytochrome P450 enzymes. The inhibitory potential of ciclesonide on cytochrome P450 isoenzymes has not been studied. In vitro studies demonstrated that the plasma protein binding of des-ciclesonide was not affected by warfarin or salicylic acid, indicating no potential for protein binding-based drug interactions.
In a drug interaction study, co-administration of orally inhaled ciclesonide and oral ketoconazole, a strong inhibitor of cytochrome P450 3A4, increased the exposure (AUC) of the active metabolite of ciclesonide, des-ciclesonide, by approximately 3.6-fold at steady state, while levels of ciclesonide remained unchanged.
In another drug interaction study, co-administration of orally inhaled ciclesonide and oral erythromycin, a moderate inhibitor of cytochrome P450 3A4, had no effect on the pharmacokinetics of either des-ciclesonide or erythromycin.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ciclesonide demonstrated no carcinogenic potential in a study of oral doses up to 900 mcg/kg (approximately 20 and 10 times the maximum human daily intranasal dose in adults and adolescents ≥ 12 years of age and children, 6 to 11 years of age, respectively, based on mcg/m2) in mice for 104 weeks and in a study of inhalation doses up to 193 mcg/kg (approximately 8 and 5 times the maximum human daily intranasal dose in adults and adolescents ≥ 12 years of age and children, 6 to 11 years of age, respectively, based on mcg/m2) in rats for 104 weeks. Ciclesonide was not mutagenic in an Ames test or in a forward mutation assay and was not clastogenic in a human lymphocyte assay or in an in vitro micronucleus test. However, ciclesonide was clastogenic in the in vivo mouse micronucleus test. The concurrent reference corticosteroid (dexamethasone) in this study showed similar findings. No evidence of impairment of fertility was observed in a reproductive study conducted in male and female rats both dosed orally up to 900 mcg/kg/day (approximately 35 times the maximum human daily intranasal dose in adults based on mcg/m2).
13.2 Animal Toxicology and Pharmacology
Reproductive Toxicology Studies: Oral administration of ciclesonide in rats up to 900 mcg/kg (approximately 35 times the maximum human daily dose in adults based on mcg/m2) produced no teratogenicity or other fetal effects. However, subcutaneous administration of ciclesonide in rabbits at 5 mcg/kg (less than the maximum daily intranasal dose in adults based on mcg/m2) or greater produced fetal toxicity. This included fetal loss, reduced fetal weight, cleft palate, skeletal abnormalities including incomplete ossifications, and skin effects. No toxicity was observed at 1 mcg/kg (less than the maximum human daily intranasal dose in adults based on mcg/m2).
14 CLINICAL STUDIES
14.1 Seasonal and Perennial Allergic Rhinitis
Adults and Adolescent Patients 12 Years of Age and Older: The efficacy of OMNARIS Nasal Spray was evaluated in 3 randomized, double-blind, parallel-group, multicenter, placebo-controlled clinical trials of 2 to 6 weeks duration conducted in the United States and Canada in adolescents and adults with allergic rhinitis. The three trials included a total of 1524 patients (495 males and 1029 females) of whom 79 were adolescents, ages 12 to 17 years. The racial distribution in these three trials included 1374 Caucasians, 69 Blacks, 31 Asians, and 50 patients classified as Other. Of the 1524 patients, 546 patients received OMNARIS Nasal Spray 200 mcg once daily administered as 2 sprays in each nostril. Patients enrolled in the studies were 12 to 86 years of age with a history of seasonal or perennial allergic rhinitis, a positive skin test to at least one relevant allergen, and active symptoms of allergic rhinitis at study entry. Assessment of efficacy in these trials was based on patient recording of four nasal symptoms (runny nose, nasal itching, sneezing, and nasal congestion) on a 0-3 categorical severity scale (0=absent, 1=mild, 2=moderate, and 3=severe) as reflective or instantaneous scores. Reflective scoring required the patients to record symptom severity over the previous 12 hours; the instantaneous scoring required patients to record symptom severity at the time of recording. The results of these trials showed that patients treated with OMNARIS Nasal Spray 200 mcg once daily exhibited statistically significantly greater decreases in total nasal symptom scores than placebo-treated patients. Secondary measures of efficacy were also generally supportive.
Dose-Ranging Trial: One of the three trials was a 2-week dose-ranging trial that evaluated efficacy of four doses of OMNARIS Nasal Spray in patients with seasonal allergic rhinitis. The primary efficacy endpoint was the difference from placebo in the change from baseline of the sum of morning and evening reflective total nasal symptom score averaged over the 2-week treatment period. Results of the primary efficacy endpoint are shown in Table 3. In this trial OMNARIS Nasal Spray 200 mcg once daily was statistically significantly different from placebo, but the lower doses were not statistically significantly different from placebo.
|
*Sum of AM and PM Scores; Maximum score = 24 |
||||||
|
** Estimates, 95% Confidence Intervals, and p-values were obtained from repeated measures ANCOVA analysis with treatment, baseline, day, and treatment by day interaction effects included in the model. |
||||||
| Treatment | N | Baseline* | Change from Baseline | Difference from Placebo** | ||
| Estimate | 95% CI | p-value | ||||
| Seasonal Allergic Rhinitis Trial – Reflective total nasal symptom score | ||||||
| Ciclesonide 200 mcg | 144 | 18.8 | -5.73 | -1.35 | (-2.43, -0.28) | 0.014 |
| Ciclesonide 100 mcg | 145 | 18.7 | -5.26 | -0.88 | (-1.96, 0.19) | 0.11 |
| Ciclesonide 50 mcg | 143 | 18.4 | -4.82 | -0.44 | (-1.52, 0.63) | 0.42 |
| Ciclesonide 25 mcg | 146 | 18.7 | -4.74 | -0.35 | (-1.42, 0.71) | 0.51 |
| Placebo | 148 | 17.8 | -4.38 | |||
Seasonal Allergic Rhinitis Trial: The second trial was a 4-week single dose level trial conducted in patients with seasonal allergic rhinitis. The primary efficacy endpoint in the seasonal allergic rhinitis trial was the difference from placebo in the change from baseline of the average of morning and evening reflective total nasal symptom score averaged over the first 2 weeks of treatment. In this trial, OMNARIS Nasal Spray 200 mcg once daily was statistically significantly different from placebo (Table 4). Statistically significant differences in the morning pre-dose instantaneous total nasal symptom score indicate that the effect was maintained over the full 24-hour dosing interval.
Perennial Allergic Rhinitis Trial: The third trial was a 6-week single dose level trial conducted in patients with perennial allergic rhinitis. The primary efficacy endpoint in the perennial allergic rhinitis trial was the difference from placebo in the change from baseline of the average of morning and evening reflective total nasal symptom score averaged over the 6 weeks of treatment. In this trial, OMNARIS Nasal Spray 200 mcg once daily was statistically significantly different from placebo (Table 4). Statistically significant differences in the morning pre-dose instantaneous total nasal symptom score indicate that the effect was maintained over the full 24-hour dosing interval.
|
*Mean of AM and PM score from reflective total nasal symptom score; Mean of AM score for instantaneous total nasal symptom score; Maximum = 12 |
||||||
|
** Estimates, 95% Confidence Intervals, and p-values were obtained from repeated measures ANCOVA analysis with treatment, baseline, day, and treatment by day interaction effects included in the model. |
||||||
| Treatment | N | Baseline* | Change from Baseline | Difference from Placebo** | ||
| Estimate | 95% CI | p-value | ||||
| Seasonal Allergic Rhinitis Trial – Reflective total nasal symptom score | ||||||
| Ciclesonide 200 mcg | 162 | 8.96 | -2.40 | -0.90 | (-1.36, -0.45) | <0.001 |
| Placebo | 162 | 8.83 | -1.50 | |||
| Seasonal Allergic Rhinitis Trial – Instantaneous total nasal symptom score | ||||||
| Ciclesonide 200 mcg | 162 | 8.45 | -1.87 | -0.84 | (-1.30, -0.39) | <0.001 |
| Placebo | 162 | 8.33 | -1.03 | |||
| Perennial Allergic Rhinitis Trial – Reflective total nasal symptom score | ||||||
| Ciclesonide 200 mcg | 232 | 7.59 | -2.51 | -0.62 | (-0.97, -0.28) | <0.001 |
| Placebo | 229 | 7.72 | -1.89 | |||
| Perennial Allergic Rhinitis Trial – Instantaneous total nasal symptom score | ||||||
| Ciclesonide 200 mcg | 232 | 7.05 | -1.99 | -0.53 | (-0.90, -0.17) | 0.004 |
| Placebo | 229 | 7.05 | -1.46 | |||
Onset of action: Onset of action was evaluated in two environmental exposure unit studies in patients with seasonal allergic rhinitis receiving a single dose of OMNARIS Nasal Spray 200 mcg. Results from these two studies did not demonstrate a replicate onset of action within the assessment period. Onset of action was also evaluated in the 4-week seasonal allergic rhinitis and in the 6-week perennial allergic rhinitis trial by frequent recording of instantaneous symptom score after the first dose. In these trials, onset of effect was seen within 24 to 48 hours with further symptomatic improvement observed over 1 to 2 weeks in seasonal allergic rhinitis and 5 weeks in perennial allergic rhinitis.
Pediatric Patients Aged 6 to 11 Years: The efficacy of OMNARIS Nasal Spray was evaluated in two randomized, double-blind, parallel-group, multicenter, placebo-controlled clinical trials in 1282 patients 6 to 11 years of age with allergic rhinitis. Of the two trials, one was 2 weeks in duration conducted in patients with seasonal allergic rhinitis that evaluated efficacy of 200 mcg and 100 mcg of OMNARIS Nasal Spray once daily. The other trial was 12 weeks in duration conducted in patients with perennial allergic rhinitis that evaluated efficacy of 200 mcg, 100 mcg, and 25 mcg of OMNARIS Nasal Spray once daily. Of the total number of patients enrolled in the 2 studies, 380 were treated with 200 mcg of OMNARIS Nasal Spray once daily. The primary efficacy endpoint was the difference from placebo in the change from baseline of the average of morning and evening reflective total nasal symptom score averaged over 2 weeks of treatment in the seasonal allergic rhinitis trial and over the first 6 weeks of treatment in the perennial allergic rhinitis trial. In the 2-week trial in patients with seasonal allergic rhinitis, the OMNARIS Nasal Spray 200 mcg once daily dose was statistically significantly different from placebo, but the 100 mcg once daily dose was not statistically significantly different from placebo. The efficacy results for the seasonal allergic rhinitis trial are shown in Table 5.
|
*Mean of AM and PM score from reflective total nasal symptom score; Maximum = 12 |
||||||
|
** Estimates, 95% Confidence Intervals, and p-values were obtained from repeated measures ANCOVA analysis with treatment, baseline, day, and treatment by day interaction effects included in the model. |
||||||
| Treatment | N | Baseline* | Change from Baseline | Difference from Placebo** | ||
| Estimate | 95% CI | p-value | ||||
| Reflective total nasal symptom score | ||||||
| Ciclesonide 200 mcg | 215 | 8.25 | -2.46 | -0.39 | (-0.76, -0.02) | 0.040 |
| Ciclesonide 100 mcg | 199 | 8.41 | -2.38 | -0.32 | (-0.69, 0.06) | 0.103 |
| Placebo | 204 | 8.41 | -2.07 | |||
In the 12-week trial in patients with perennial allergic rhinitis, none of the ciclesonide doses were statistically significantly different from placebo. The means and 95% confidence intervals for the differences (OMNARIS Nasal Spray minus placebo) between OMNARIS Nasal Spray 200 mcg, 100 mcg, and 25 mcg treatment groups and placebo were -0.31 (-0.75, 0.13), 0.02 (-0.41, 0.46), and 0.09 (-0.35, 0.53), respectively.
Pediatric Patients Aged 2 to 5 Years: Efficacy of OMNARIS Nasal Spray in patients 2 to 5 years of age has not been established [see Pediatric Use (8.4)].
16 HOW SUPPLIED/STORAGE AND HANDLING
OMNARIS is supplied in an amber glass bottle and provides for nasal delivery with a manual metered pump. OMNARIS Nasal Spray is supplied with an oxygen absorber sachet and enclosed in a foil pouch. The contents of one 12.5 gram bottle provide 120 actuations, after initial priming. Each spray delivers 50 mcg of ciclesonide from the nasal actuator. Prior to initial use, OMNARIS Nasal Spray must be gently shaken and then the pump must be primed by actuating eight times. The OMNARIS Nasal Spray bottle has been filled with an excess to accommodate the priming activity. The bottle should be discarded after removal from the foil pouch either after 120 sprays following initial priming (since the amount of ciclesonide delivered per spray thereafter may be substantially less than the labeled dose) or after 4 months. Patient instructions are also provided.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature]. Do not freeze. Shake gently before use. Keep out of reach of children.
Omnaris Nasal Spray 50 mcg, 120 metered sprays; net fill weight 12.5 g.
NDC 63402-701-01
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use).
17.1 Local Nasal Effects
Patients should be informed that treatment with OMNARIS Nasal Spray may lead to adverse reactions, which include epistaxis and nasal ulceration. Candida infection may also occur with treatment with OMNARIS Nasal Spray. In addition, nasal corticosteroids are associated with nasal septal perforation and impaired wound healing. Avoid spraying OMNARIS Nasal Spray directly onto the nasal septum. Patients who have experienced recent nasal ulcers, nasal surgery, or nasal trauma should not use OMNARIS Nasal Spray until healing has occurred [see Warnings and Precautions (5.1)].
17.2 Cataracts and Glaucoma
Patients should be informed that glaucoma and cataracts are associated with nasal and inhaled corticosteroid use. The patient should inform his/her health care provider if a change in vision is noted while using OMNARIS Nasal Spray [see Warnings and Precautions (5.2)].
17.3 Immunosuppression
Patients who are on immunosuppressive doses of corticosteroids should be warned to avoid exposure to chickenpox or measles, and if exposed, to consult their physician without delay. Patients should be informed of potential worsening of existing tuberculosis, fungal, bacterial, viral or parasitic infections, or ocular herpes simplex [see Warnings and Precautions (5.3)].
17.4 Use Daily
Patients should use OMNARIS Nasal Spray at regular intervals since its effectiveness depends on its regular use. In clinical trials, the onset of effect was seen within 24 to 48 hours with further symptomatic improvement observed over 1 to 2 weeks in seasonal allergic rhinitis and 5 weeks in perennial allergic rhinitis. Initial assessment of response should be made during this time frame and periodically until the patient's symptoms are stabilized. The patient should take the medication as directed and should not exceed the prescribed dosage. The patient should contact the physician if symptoms do not improve by a reasonable time or if the condition worsens.
17.5 Keep Spray Out of Eyes
Patients should be informed to avoid spraying OMNARIS Nasal Spray in their eyes.
17.6 Storage and Handling
It is important that the bottle is gently shaken prior to use to ensure that a consistent amount is dispensed per actuation. The bottle should be discarded after 120 actuations following initial priming or after 4 months after the bottle is removed from the foil pouch, whichever occurs first.
SUNOVION
Manufactured for
Sunovion Pharmaceuticals Inc.
Marlborough MA 01752 USA
Made in Germany
© 2010, 2013 Sunovion Pharmaceuticals Inc. All rights reserved
OMNARIS is a registered trademark of Takeda GmbH and is used under license.
For customer service, call 1-888-394-7377.
To report adverse events, call 1-877-737-7226.
For medical information, call 1-800-739-0565.
March 2013
901379R03
Patient Information
OMNARIS® [Ŏm-nĕ'-rĭs] (ciclesonide) Nasal Spray, 50 mcg
Important Note: For Intranasal Use Only. Avoid spraying in eyes or directly onto the nasal septum (the wall between the two nostrils).
Read this leaflet before you start using OMNARIS Nasal Spray and each time you refill your prescription. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions about OMNARIS Nasal Spray, ask your healthcare provider or pharmacist.
What is OMNARIS Nasal Spray?
OMNARIS Nasal Spray contains a medicine called ciclesonide, which is a synthetic corticosteroid (a substance that reduces inflammation). This medicine is used to treat nasal symptoms (i.e., runny nose, itchy nose, sneezing, and nasal congestion) that happen with:
- Seasonal nasal allergy in adults and children 6 years of age and older.
- Year-round nasal allergy symptoms in adults and adolescents 12 years of age and older.
How do I use OMNARIS Nasal Spray?
Use your nasal spray exactly as prescribed by your healthcare provider.
Seasonal allergy symptoms may improve over 1 to 2 weeks; year-round allergy symptoms may improve over 5 weeks. If your symptoms do not improve or get worse, call your healthcare provider.
Dosage
- OMNARIS Nasal Spray is used 1 time each day, 2 sprays in each nostril.
- Do not use more than a total of 2 sprays in each nostril each day.
What are the side effects of OMNARIS Nasal Spray?
Common side effects with OMNARIS Nasal Spray include:
- Headache
- Nose bleeds
- Stuffy nose
- Ear pain
- Sore throat
- Dizziness
What are the other risks of using OMNARIS Nasal Spray?
- Nasal fungal infection.
- Slow healing of wounds. Do not use OMNARIS Nasal Spray until your nose has healed if: you have a sore in your nose, you have had surgery on your nose, or your nose has been injured.
- Eye problems, including glaucoma and cataracts. You should have regular eye exams.
- Immune system effects may increase your risk of infection. You should avoid being around people with Chicken Pox or Measles.
- Slow growth in children. A child taking OMNARIS Nasal Spray should have his/her growth checked regularly.
These are not all the possible side effects of OMNARIS Nasal Spray. Tell your healthcare provider about any side effects that bother you or do not go away.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What are the ingredients in OMNARIS Nasal Spray?
Active ingredient: ciclesonide
Inactive ingredients: purified water, microcrystalline cellulose, carboxymethylcellulose sodium, hypromellose, potassium sorbate and edetate sodium; and hydrochloric acid to adjust the pH to 4.5.
This leaflet does not contain all of the information about your medicine. If you have any questions ask your healthcare provider or pharmacist.
You may want to read this leaflet again. Please DO NOT THROW IT AWAY until you have finished your medicine.
Patient's Instructions for Use
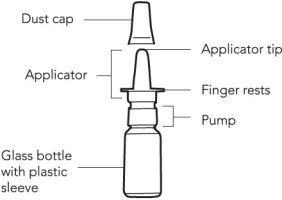
OMNARIS Nasal Spray is supplied in an amber glass bottle in a protective plastic sleeve and should be handled with care.
Preparing For Use
- Remove OMNARIS Nasal Spray from its foil pouch. Count 4 months from today and write this new date on the sticker on the carton (the date that is 4 months after removing the bottle from the foil pouch). Peel off the sticker and place it in the space on your nasal spray bottle. It is important that you throw away the nasal spray bottle after this date.
- Priming OMNARIS Nasal Spray. Before you use OMNARIS Nasal Spray for the first time, you will need to prime the bottle. Hold the bottle upright and shake the bottle gently. To prime OMNARIS Nasal Spray, fully press down on the finger rests of the applicator eight times (See Figure 1 and Figure 2). If you do not use the nasal spray for 4 days, you will need to shake the bottle gently, and prime the pump again by spraying one time, or until you see a fine mist.
Figure 1
Using the Spray
- Blow your nose to clear your nostrils, if needed.
- Shake the bottle gently and remove the dust cap (See Figure 1).
- Hold the bottle firmly with your index and middle finger on either side of the applicator (on finger rests) while supporting the base of the bottle with your thumb (See Figure 2).
Figure 2
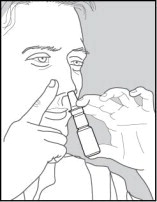
- Insert applicator tip into one nostril, and close the other nostril with your finger (See Figure 3).
Figure 3
- Tilt your head forward slightly. Keep the bottle upright, and press the finger rests quickly and firmly to activate the pump. Breathe in (inhale) through your nose as you spray (See Figure 4). Try not to get any spray in your eyes or directly on your nasal septum (the wall between the two nostrils).
Figure 4
- Repeat steps 3-5 for the second spray in the same nostril and for each spray in the other nostril.
How do I store OMNARIS Nasal Spray?
- Keep OMNARIS Nasal Spray clean and dry at all times.
- Store OMNARIS Nasal Spray between 59° F and 86° F.
- Do not freeze.
- Keep OMNARIS Nasal Spray and all medicines out of the reach of children.
How Do I Know When the OMNARIS Nasal Spray Bottle Is Empty?
Each bottle of OMNARIS Nasal Spray contains enough medicine for you to spray medicine from the bottle 120 times. Do not use a bottle of OMNARIS Nasal Spray after 120 sprays (not counting the priming sprays) have been used or after the “discard by date” you wrote on the sticker when you opened the foil pouch. You may still see some medicine in the bottle. Talk with your healthcare provider before your supply of OMNARIS Nasal Spray runs out to see if you should get a refill of your medicine.
Applicator Cleaning Instructions
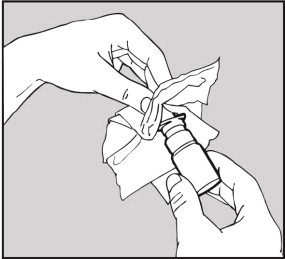
Wipe the applicator tip with a clean tissue and replace the dust cap, after you use your nasal spray each day. (See Figure 5)
Figure 5
If the applicator is clogged or needs more thorough cleaning, use the following cleaning instructions (Do not try to unblock the tiny spray hole on the applicator with a pin or other sharp object. Do not twist or try to remove the white plastic pump attached to the medicine bottle.):
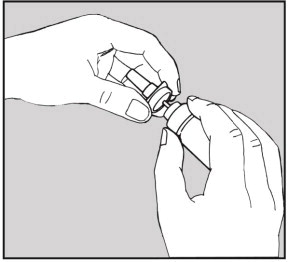
- Remove the dust cap, hold the white plastic pump firmly with one hand and then carefully pull upwards to free the applicator. (See Figure 6)
Figure 6
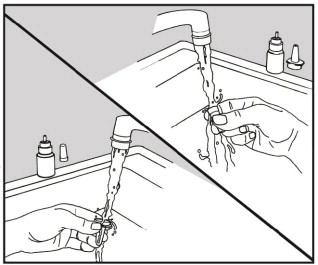
- Wash the dust cap and applicator with warm water. (See Figure 7)
Figure 7
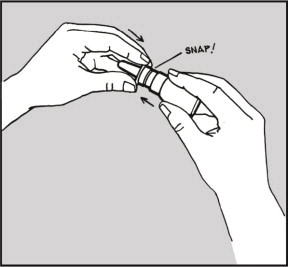
- Dry the applicator, and put it back on the bottle. The applicator will snap into place when properly positioned. (See Figure 8)
Figure 8
- Prime the unit with one spray or until you see a fine mist.
- Put the dust cap back on the applicator.
SUNOVION
Manufactured for
Sunovion Pharmaceuticals Inc.
Marlborough, MA 01752 USA
Made in Germany
© 2010, 2013 Sunovion Pharmaceuticals Inc. All rights reserved.
OMNARIS is a registered trademark of Takeda GmbH and is used under license.
For customer service, call 1-888-394-7377
Revised: March 2013
901380R02
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 120 ACTUATIONS LABEL
NDC 63402-701-01
Net Contents: 12.5 g
OMNARIS®
(ciclesonide) Nasal Spray, 50 mcg
120 Metered Actuations
FOR INTRANASAL USE ONLY
Rx only
SUNOVION
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 120 ACTUATION POUCH
NDC 63402-701-01
Net Contents: 12.5 g
OMNARIS®
(ciclesonide) Nasal Spray, 50 mcg
120 Metered Actuations
FOR INTRANASAL USE ONLY
Rx only
Discard OMNARIS® 4 months after opening foil pouch
Shake gently before use
SUNOVION
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 120 ACTUATION CARTON
NDC 63402-701-01
Net Contents: 12.5 g
OMNARIS®
(ciclesonide) Nasal Spray, 50 mcg
120 Metered Actuations
FOR INTRANASAL USE ONLY
Rx only
FRAGILE: Glass bottle inside.
Attention Pharmacist:
Dispense with accompanying Patient's Instructions for Use.
Attention Patient:
See Patient's Instructions for Use and cleaning
instructions before using this product.
Discard OMNARIS® 4 months after opening the foil pouch.
SUNOVION
| OMNARIS
ciclesonide spray |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Sunovion Pharmaceuticals Inc. (131661746) |