Label: EBANGA- ansuvimab kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 80673-001-01, 80673-001-36, 80673-777-01, 80673-777-04, view more80673-777-08 - Packager: Ridgeback Biotherapeutics, LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EBANGA™ safely and effectively. See full prescribing information for EBANGA.
EBANGA (ansuvimab-zykl) for injection, for intravenous use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
EBANGA™ (ansuvimab-zykl) is a Zaire ebolavirus glycoprotein (EBOV GP)-directed human monoclonal antibody indicated for the treatment of infection caused by Zaire ebolavirus in adult and pediatric patients, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection. ( 1)
Limitation of Use
- The efficacy of EBANGA has not been established for other species of the Ebolavirus and Marburgvirus genera .
- Zaire ebolavirus can change over time, and factors such as emergence of resistance or changes in viral virulence could diminish the clinical benefit of antiviral drugs. Consider available information on drug susceptibility patterns for circulating Zaire ebolavirus strains when deciding whether to use EBANGA.
DOSAGE AND ADMINISTRATION
The recommended dose of EBANGA for adult and pediatric patients is 50 mg/kg reconstituted, further diluted, and administered as a single intravenous infusion over 60 minutes. ( 2.1, 2.2)
See Full Prescribing Information for instructions on preparation, dilution and administration of EBANGA injection. ( 2.2)
DOSAGE FORMS AND STRENGTHS
For injection: 400 mg lyophilized powder in a single-dose vial for reconstitution and further dilution. ( 3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions Including Infusion-Associated Events: Hypersensitivity reactions including infusion-associated events have been reported with EBANGA. These may include acute, life-threatening reactions during and after the infusion. Monitor patients and in the case of severe or life-threatening hypersensitivity reactions, discontinue the administration of EBANGA immediately and administer appropriate emergency care. ( 5.1)
ADVERSE REACTIONS
The most frequently reported adverse events (≥ 5%) after administration of EBANGA were pyrexia, tachycardia, diarrhea, vomiting, hypotension, tachypnea, and chills. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ridgeback Biotherapeutics, LP at 1-833-846-3789 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Interaction with live vaccine indicated for prevention of Zaire ebolavirus infection: No vaccine interaction studies have been performed. EBANGA may reduce the efficacy of the live vaccine. The interval between administration of EBANGA therapy and live vaccination should be in accordance with current vaccination guidelines. ( 7.1)
USE IN SPECIFIC POPULATIONS
Lactation: Patients infected with Zaire ebolavirus should be instructed not to breastfed due to the potential for Zaire ebolavirus transmission. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult and Pediatric Patients
2.2. Preparation, Administration, and Storage Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions Including Infusion-Associated Events
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Vaccine Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED / STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
EBANGA is indicated for the treatment of infection caused by Zaire ebolavirus in adult and pediatric patients, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection [see Dosage and Administration (2.2) and Clinical Studies (14)] .
Limitations of Use:
The efficacy of EBANGA has not been established for other species of the Ebolavirus and Marburgvirus genera.
Zaire ebolavirus can change over time, and factors such as emergence of resistance, or changes in viral virulence could diminish the clinical benefit of antiviral drugs. Consider available information on drug susceptibility patterns for circulating Zaire ebolavirus strains when deciding whether to use EBANGA.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult and Pediatric Patients
The recommended dosage of EBANGA is 50 mg/kg administered as a single intravenous (IV) infusion over 60 minutes. EBANGA must be reconstituted with Sterile Water for Injection, USP then further diluted in 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP prior to IV infusion [see Dosage and Administration (2.2)] .
2.2. Preparation, Administration, and Storage Instructions
EBANGA must be prepared and administered under the supervision of a health care professional.
Reconstitution Instructions
- Aseptically reconstitute and further dilute EBANGA prior to IV infusion. Do not administer as an IV push or bolus.
- More than one vial may be needed for a full dose. Calculate the dose (mg) based on the patient's actual weight in kg and the number of EBANGA vials required [see Dosage and Administration (2.1)] .
- Prior to reconstitution, allow EBANGA vial(s) to reach ambient temperature (15°C to 27°C [59°F to 81°F]) for approximately 20 minutes. If for any reason reconstitution cannot proceed immediately upon reaching ambient temperature, vials that have NOT been reconstituted may be kept at ambient temperature, protected from light, for no more than 24 hours.
- Immediately upon reaching ambient temperature, use a sterile 10 mL syringe and an 18-gauge needle to withdraw 7.7 mL of Sterile Water for Injection, USP. Insert the needle tip into the EBANGA vial. Holding horizontally, angle the needle down at an approximate 45° angle, above the lyophilized powder, which has a cake-like appearance. Slowly inject the diluent along the wall of the vial and without any air to avoid foaming and bubbles.
- Gently swirl (do NOT shake) for approximately 10 seconds; then set the vial down to rest for at least 10 seconds. Repeat until the cake is dissolved. This may take up to 20 minutes.
- Upon reconstitution, one vial delivers 8 mL of solution that is clear to slightly opalescent and colorless to slightly yellow containing 50 mg/mL of ansuvimab-zykl. Do NOT administer and discard the vial if the reconstituted solution is discolored or contains visible particles.
- Dilute the EBANGA solution immediately upon reconstitution. If needed, the reconstituted solution may be stored refrigerated at 2°C to 8°C (36°F to 46°F), protected from light, for up to 4 hours. This 4-hour window includes time required for further dilution and EBANGA solution should be infused immediately upon further dilution.
Dilution Instructions
- Following reconstitution, EBANGA must be further diluted prior to IV infusion.
- Use an 18-20 gauge, 1-1.5" needle with an appropriately sized syringe up to 60 mL to perform the dilution steps.
- Prepare the EBANGA IV dosing solution using an appropriately sized syringe up to 60 mL.
- For patients weighing ≥ 2 kg, prepare the diluent using either a latex-free, di-ethylhexylphthalate (DEHP)-free 0.9% Sodium Chloride Injection USP infusion bag, or latex-free, DEHP-free 5% Dextrose Injection USP infusion bag. For patients weighing 0.5 to < 2 kg use a pump-compatible syringe (Table 1).
- For adult and pediatric patients, either 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP can be used as the diluent.
- The total volume of the infusion solution to be administered is based on the patient's body weight and is specified in Table 1.
- Use a 10 mL syringe compatible with the IV infusion pump.
- Fill the 10 mL syringe with the appropriate amount of diluent (Table 1).
- Add the calculated volume of EBANGA to the 10 mL syringe (Table 1).
- Mix the diluted solution by gentle inversion (3 to 5 times) until admixed. Do not shake.
- Select a diluent solution infusion bag size of appropriate fill volume based on the patient's body weight (see Table 1).
- Withdraw and discard from the bag a volume of diluent solution that will leave remaining in the bag the appropriate volume based on the patient's weight (see
Table 1). Then add the calculated volume of EBANGA to the bag based on the patient's weight (see
Table 1).
For example, for a 55 kg patient, withdraw and discard 150 mL of diluent from a 250 mL infusion bag. Then add 55 mL of EBANGA to obtain a total infusion volume of 155 mL. - Gently invert the IV bag 5 to 10 times until the diluted solution is admixed. Do NOT shake.
- Infuse the EBANGA solution immediately upon dilution. If needed, the diluted infusion solution may be stored refrigerated at 2°C to 8°C (36°F to 46°F), for up to 4 hours. Do not freeze the diluted solution. If refrigerated, allow approximately 20 minutes for the diluted solution to come to ambient temperature prior to use. These time limits include reconstitution time.
Prepare a medical label including patient weight in kg, date, and time of reconstitution. - Discard vial(s) and all unused contents.
Table 1: EBANGA Volume, Diluent Volume and Total Infusion Volume by Body Weight Weight in kg Volume of EBANGA Diluent Volume (mL) *,† Final Infusion Volume (mL) Syringe or Infusion Bag Volume for IV Administration 0.5 kg 1 mL/kg 2.5 mL 3 mL 10 mL syringe compatible with IV infusion pump 1 kg 5 mL 6 mL 2 to 10 kg 10 mL 12 to 20 mL 25 mL IV bag 11 to 25 kg 25 mL 36 to 50 mL 50 mL IV bag 26 to 50 kg 50 mL 76 to 100 mL 100 mL IV bag 51 to 100 kg 100 mL 151 to 200 mL 250 mL IV bag 101 kg and above 150 mL 251 mL and above 500 mL IV bag Administration
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not administer if discolored or if the vial contains visible particles.
- Do not mix with or administer as an infusion with other medicinal products.
- Prepare the IV infusion line with 1.2 micron in-line filter extension set.
- Administer the IV infusion solution over 60 minutes.
- The diluted EBANGA IV solution can be infused via a central line or peripheral catheter. Do not administer EBANGA as an IV push or bolus.
- Do not co-administer other drugs simultaneously through the same infusion line.
- Infusions may be slowed or stopped if necessary, to alleviate any side effects.
- At the end of the infusion, if a syringe pump was used, then remove the syringe and flush the line with 2 to 5 ml of diluent, but not to exceed the total infusion volume. If an infusion bag was used, replace the empty bag and flush the line by infusing at least 25 mL of the diluent, to ensure complete product administration.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions Including Infusion-Associated Events
Hypersensitivity reactions including infusion-associated events have been reported with EBANGA. These may include acute, life-threatening reactions during and after the infusion. Monitor all patients for signs and symptoms including, but not limited to, hypotension, chills and elevation of fever, during and following EBANGA infusion. In the case of severe or life-threatening hypersensitivity reactions, discontinue the administration of EBANGA immediately and administer appropriate emergency care [see Adverse Reactions (6.1)].
Infusion could not be completed in 1% of subjects who received EBANGA due to infusion-associated adverse events. The rate of infusion of EBANGA may be slowed or interrupted if the patient develops any signs of infusion-associated events or other adverse events [see Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions Including Infusion-Associated Events [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice.
Overall, 424 adult and pediatric subjects with Zaire ebolavirus infection received EBANGA in one clinical trial and as part of an expanded access program during the 2018 Zaire ebolavirus outbreak in the Democratic Republic of Congo (DRC).
In the PALM trial, the safety of EBANGA was evaluated in a multi-center, open-label, randomized controlled trial, in which 173 subjects (119 adults and 54 pediatric subjects) with confirmed Zaire ebolavirus infection received EBANGA as a single 50 mg/kg IV infusion and 168 subjects received an investigational control [see Clinical Studies (14)] . All subjects received optimized standard of care treatment (oSOC). The median age of the study population that received EBANGA was 26 years (range: 1 day to 85 years). Fifty-five percent (55%) of enrolled subjects were female and 45% were male.
During the same outbreak, 251 subjects (173 adults and 78 pediatric subjects) with laboratory-confirmed Zaire ebolavirus infection received EBANGA under an expanded access program; 57% of whom were female and 43% of whom were male. Ages ranged from 6 days to 80 years, with a median age of 25 years.
Common Adverse Events
Table 2 summarizes the adverse events that were reported in the PALM trial from a pre-defined list of signs and symptoms that occurred during EBANGA infusion. The evaluation of adverse events in subjects who received EBANGA may have been confounded by the signs and symptoms of the underlying Zaire ebolavirus infection. Twenty nine percent (n=51) of subjects who received EBANGA in the PALM Trial experienced a pre-specified infusion-related adverse event. The most common pre-specified infusion-related adverse event reported in at least 10% of subjects who received EBANGA was fever (Table 2). The adverse event profile in adult and pediatric subjects treated with EBANGA was similar.
Table 2: Adverse Events That Occurred During Infusion in >10% of Adult and Pediatric Subjects in the PALM Trial Adverse Event * EBANGA
(N=173)
%Control †
(N=168)
%- *
- Adverse events in this table were reported on the day of infusion, and included signs and symptoms that occurred during or immediately after infusion
- †
- Investigational therapy administered as three separate infusions
- ‡
- Adverse events that occurred during infusion but were not pre-specified.
- §
- The term chills includes other similar adverse events including rigors and tremors
Pyrexia 17 58 Tachycardia 9 32 Diarrhea ‡ 9 18 Vomiting ‡ 8 23 Hypotension 8 31 Tachypnea 6 28 Chills § 5 33 Hypoxia ‡, 3 11 The following pre-specified symptoms, which were assessed on a daily basis during admission while admitted to the treatment unit, were reported in ≥40% of subjects who received EBANGA: diarrhea, pyrexia, abdominal pain, and vomiting. Evaluation of these symptoms may have been confounded by the underlying Zaire ebolavirus infection.
Discontinuation and Infusion Rate Adjustments
Approximately 99% of subjects who received EBANGA in the PALM trial were able to complete their dose within one hour. Two subjects who received EBANGA (1%) did not receive their complete infusion. In eight subjects (5%) the EBANGA infusion rate was decreased due to an AE [see Warnings and Precautions (5.1)] .
Selected Laboratory Abnormalities in the PALM Trial
Table 3 presents selected laboratory abnormalities (worsening to Grade 3 or 4 compared to baseline) in the PALM trial.
Table 3: Selected Grade 3 and 4 Laboratory Abnormalities a, Worsened Grade from Baseline in the PALM Trial Laboratory Test * EBANGA
N=173
%Control
N=168
%ULN= upper limit of normal Sodium, high ≥ 154 mmol/L 5 4 Sodium, low < 125 mmol/L 7 11 Potassium, high ≥ 6.5 mmol/L 15 12 Potassium, low < 2.5 mmol/L 6 8 Creatinine (mg/dL) > 1.8 × ULN or ≥ 1.5 × baseline † 27 23 Alanine aminotransferase (U/L) ≥ 5 × ULN ‡ 12 14 Aspartate aminotransferase (U/L) ≥ 5 × ULN § 13 18 -
7 DRUG INTERACTIONS
7.1 Vaccine Interactions
No vaccine-therapeutic interaction studies have been performed in human subjects using EBANGA. However, because of the potential for EBANGA to inhibit replication of a live vaccine virus indicated for prevention of Zaire ebolavirus infection and possibly reduce the efficacy of the vaccine, avoid the concurrent administration of a live vaccine during treatment with EBANGA. The interval between administration of EBANGA therapy and live vaccination should be in accordance with current vaccination guidelines. The efficacy of EBANGA among subjects who reported receipt of a recombinant live vaccine prior to their enrollment in the PALM trial was similar to subjects who did not report receiving a vaccine prior to enrollment.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Zaire ebolavirus infection is life-threatening for both the mother and fetus and treatment should not be withheld due to pregnancy (see Clinical Considerations) . Available data from the PALM trial in which pregnant women with Zaire ebolavirus infection were treated with EBANGA demonstrate the high rate of maternal and fetal/neonatal morbidity consistent with published literature regarding the risk associated with underlying maternal Zaire ebolavirus infection. These data are insufficient to evaluate for a drug associated risk of major birth defects, miscarriage, or adverse maternal/fetal outcome.
Animal reproduction studies with ansuvimab-zykl have not been conducted. Monoclonal antibodies, such as EBANGA, are transported across the placenta; therefore, EBANGA has the potential to be transferred from the mother to the developing fetus.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Maternal, fetal and neonatal outcomes are poor among pregnant women infected with Zaire ebolavirus. The majority of such pregnancies result in maternal death with miscarriage, stillbirth, or neonatal death. Treatment should not be withheld due to pregnancy.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that mothers with confirmed Zaire ebolavirus not breastfeed their infants to reduce the risk of postnatal transmission of Zaire ebolavirus infection.
There are no data on the presence of ansuvimab-zykl in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to ansuvimab-zykl are unknown.
8.4 Pediatric Use
The safety and effectiveness of EBANGA for the treatment of infection caused by Zaire ebolavirus have been established in pediatric patients from birth to less than 18 years of age. Use of EBANGA for this indication is supported by evidence from a multi-center, open label, randomized controlled trial of EBANGA in adults and pediatric subjects that included 54 pediatric subjects birth to less than 18 years of age, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection. Of the total number of subjects administered EBANGA in the PALM trial, pediatric subjects (1 day to 17 years) accounted for 31% (n=54) of the study population in the PALM trial. The 28-day mortality and safety in adult and pediatric subjects treated with EBANGA were similar [see Adverse Reactions (6.1), and Clinical Studies (14)]. An additional 78 (31%) pediatric subjects from birth to less than 18 years of age received EBANGA in an expanded access program.
8.5 Geriatric Use
Clinical trials of EBANGA did not include sufficient numbers of subjects aged 65 and over to determine whether the safety profile of EBANGA is different in this population compared to younger subjects. Of the total number of subjects administered EBANGA in the PALM trial, 6 subjects (3%) were 65 years or older. The limited clinical experience has not identified differences in responses between the elderly and younger subjects.
-
11 DESCRIPTION
Ansuvimab-zykl is a Zaire ebolavirus (EBOV) glycoprotein 1 (GP1)-directed recombinant, human IgG1 monoclonal antibody. Ansuvimab-zykl is produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology and has an approximate molecular weight of 147 kDa.
EBANGA (ansuvimab-zykl) for injection is a sterile, preservative-free, off-white to white lyophilized powder in a single-dose vial for IV use after reconstitution and dilution. Each single-dose vial delivers 400 mg of ansuvimab-zykl, and L-histidine (12.4 mg), L-histidine HCl (16.8 mg), polysorbate 80 (1.6 mg), and sucrose (657 mg). After reconstitution with 7.7 mL of Sterile Water for Injection, USP, each vial delivers 8 mL of a clear and colorless to slightly yellow solution containing 50 mg/mL of ansuvimab-zykl, with an approximate pH of 6.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ansuvimab-zykl is a recombinant human monoclonal antibody with antiviral activity against Zaire ebolavirus [see Microbiology (12.4)].
12.2 Pharmacodynamics
Ansuvimab-zykl exposure-response relationship and the time course of pharmacodynamic response is unknown.
12.3 Pharmacokinetics
Limited data from 18 healthy subjects 22 to 56 years of age suggests that the pharmacokinetic profile of ansuvimab-zykl is consistent with the profile of other IgG1 monoclonal antibodies.
Pharmacokinetic data are not available for Zaire ebolavirus infected patients.
12.4 Microbiology
Mechanism of Action
EBANGA (ansuvimab-zykl) is a recombinant, human IgG1κ monoclonal antibody that binds to the glycan cap and inner chalice of the EBOV GP1 subunit. The epitope to which it binds is located within the receptor binding domain of EBOV consisting of amino acids LEIKKPDGS (GP residues 111–119).
Ansuvimab-zykl binds EBOV GP without the mucin domain with a K D of 0.2 nM at pH 7.4 and 0.6 nM at pH 5.3 as measured by biolayer interferometry. Ansuvimab-zykl blocks binding of EBOV GP1 to the Neiman Pick cell receptor 1 in host cells (IC 50 value of 0.09 μg/mL), inhibiting virus entry into the host cell. Ansuvimab-zykl exhibited Fc-mediated Antibody Dependent Cellular Cytotoxicity (ADCC) activity against cells expressing EBOV GP when effector cells were added.
Antiviral Activity
In a live virus plaque-reduction neutralization assay performed in Vero E6 cells, ansuvimab-zykl neutralized Zaire ebolavirus Mayinga with an EC 50 value of 0.06 µg/mL. In an EBOV GP lentivirus infectivity assay using HEK293 cells, ansuvimab-zykl inhibited Zaire ebolavirus Mayinga with an EC 50 value of 0.09 μg/mL and Zaire ebolavirus Makona with an EC 50 value of 0.15 μg/mL. The ADCC activity of ansuvimab-zykl was assessed in EBOV GP-transduced and non-transduced HEK293T target cells in the presence of antibody with effector cells added at an effector-to-target cell ratio 1:50 and analyzed via flow cytometry. Ansuvimab-zykl mediated ADCC, with maximal activity observed at a mAb concentration of 0.03 μg/mL. Treatment of Zaire ebolavirus infected rhesus macaques with a single IV dose of ansuvimab-zykl (50 mg per kg) generally protected infected animals from Zaire ebolavirus mediated death when drug was administered 5 days post-infection.
Resistance
No nonclinical or clinical studies evaluating resistance to ansuvimab-zykl have been conducted. The possibility of resistance to ansuvimab-zykl should be considered in patients who either fail to respond to therapy or who develop relapse of disease after an initial period of responsiveness.
Immune Response
Interaction studies with recombinant live EBOV vaccines and EBANGA have not been conducted [see Drug Interactions (7.1)] .
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of EBANGA has been evaluated in 174 subjects with confirmed Zaire ebolavirus infection in the PALM trial, a multi-center, open-label, randomized, controlled trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID; NCT03719586). The trial was conducted in the North Kivu and Ituri provinces in the Democratic Republic of Congo, where an outbreak began in August 2018, and enrolled 681 subjects of all ages, including pregnant women, with documented Zaire ebolavirus infection and symptoms of any duration who were receiving oSOC. Subjects were randomized to receive EBANGA 50 mg/kg IV as a single infusion, an investigational control 50 mg/kg IV every third day, for a total of 3 doses, or other investigational drugs. Eligible subjects had a positive reverse transcriptase-polymerase chain reaction (RT-PCR) for the nucleoprotein (NP) gene of Zaire ebolavirus and had not received other investigational treatments (with the exception of experimental vaccines) within the previous 30 days. Neonates ≤7 days of age were eligible if the mother had documented infection. Neonates born to a mother who had cleared Zaire ebolavirus following a course of her assigned investigational medication were also eligible to be enrolled at investigator discretion regarding the likelihood that the neonate was infected. Randomization was stratified by reverse transcription-PCR cycle threshold calculated using NP targets (CtNP ≤22.0 vs >22.0; corresponding to high and low viral load, respectively) and Ebola Treatment Unit (ETU) site. All subjects received oSOC consisting, at a minimum, of IV fluids, daily clinical laboratory testing, correction of hypoglycemia and electrolyte imbalances, and broad-spectrum antibiotics and antimalarials, as indicated.
The primary efficacy endpoint was 28-day mortality. The primary analysis population includes all subjects who were randomized and concurrently eligible to receive either EBANGA or the investigational control during the same time period of the trial.
The demographics and baseline characteristics are provided in Table 4 below.
Table 4: Demographics and Baseline Characteristics in PALM Trial Parameter EBANGA
N=174
N (%)Control
N=168
N (%)CtNP = cycle threshold calculated using NP targets; IQR=interquartile range; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; ETU=Ebola treatment unit. - *
- Pregnancy positive test was calculated based on subjects who were pregnant. Denominator for percentages is the number of females in the treatment group.
Mean age (years) 27.3 29.9 Age <1 month, n (%) 4 (2) 2 (1) Age 1 month to <1 year, n (%) 7 (4) 5 (3) Age 1 year to <6 years, n (%) 15 (9) 12 (7) Age 6 years to <12 years, n (%) 13 (7) 5 (3) Age 12 years to <18 years, n (%) 15 (9) 9 (5) Age 18 years to <50 years, n (%) 93 (53) 114 (68) Age 50 years to <65 years, n (%) 21 (12) 18 (11) Age ≥65 years, n (%) 6 (3) 3 (2) Female, n (%) 98 (56) 87 (52) Positive result on pregnancy test *, n (%) 5/98 (5) 4/87 (5) RT-PCR CtNP cycle threshold ≤22, n (%) 73 (42) 70 (42) Median RT-PCR CtNP (IQR) 23.3 (19.7, 28.5) 23.1 (19.0, 26.5) Median creatinine (IQR) 0.9 (0.6, 2.4) 1.2 (0.8, 4.3) Median AST (IQR) 234 (66, 978) 351 (112, 1404) Median ALT (IQR) 168 (44, 551) 236 (48, 631) Median days from onset of symptoms to randomization (IQR) 5 (3, 7) 5 (3, 7) Reported Vaccination with rVSV-ZEBOV vaccine, n (%) 36 (21) 41 (24) <10 days before ETU admission, n (%) 22/36 (61) 21/41 (51) ≥10 days before ETU admission, n (%) 12/36 (33) 18/41 (44) Timing unknown, n (%) 2/36 (6) 2/41 (5) The PALM trial was stopped early on the basis of a pre-specified interim analysis showing a statistically significant reduction in mortality for EBANGA compared to control assessed at Day 28.
Mortality efficacy results are shown in Table 5 and Figure 1.
Table 5: Mortality Rates in PALM Trial Efficacy Endpoints EBANGA *
N=174Control *
N=168N=Number of subjects in the Concurrent Intention-to-Treat population and treatment group; n=Number of subjects with the 28-day outcome. Denominator for percentages is the total number of subjects in the specific group. - *
- Both EBANGA and Control were administered with optimized standard of care
- †
- 95% CI for Difference = 95% confidence intervals were computed by inverting two one-sided exact tests.
- ‡
- The result is significant according to the interim stopping boundary, p<0.028.
- §
- Cepheid GeneXpert Ebola® Assay used for detection of Zaire ebolavirus RNA
Overall 28-day mortality, n (%) 61 (35%) 83 (49%) Mortality rate difference relative to control (95% CI) † -14.3 (-24.7, -3.7) p-Value ‡ 0.008 Baseline Viral Load High viral load (CtNP ≤ 22)§ 28-day mortality, n (%) 51/73 (70%) 60/70 (86%) Mortality rate difference relative to control (95% CI) † -15.9 (-31.6, 0.9) Low viral load (CtNP > 22)§ 28-day mortality, n (%) 10/101 (10%) 23/97 (24%) Mortality rate difference relative to control (95% CI) † -13.8 (-27.3, 0.3) Age group, 28-day mortality, n/N (%) Adults (age ≥18 years) 41/120 (34%) 68/135 (50%) 12 to < 18 years of age 5/15 (33%) 5/9 (56%) 6 to < 12 years of age 4/13 (31%) 2/5 (40%) < 6 years of age 11/26 (42%) 8/19 (42%) Sex, 28-day mortality, n/N (%) Male 30/76 (39%) 32/81 (40%) Female 31/98 (32%) 51/87 (59%) Figure 1: Kaplan-Meier Curve for Overall Mortality in PALM Trial
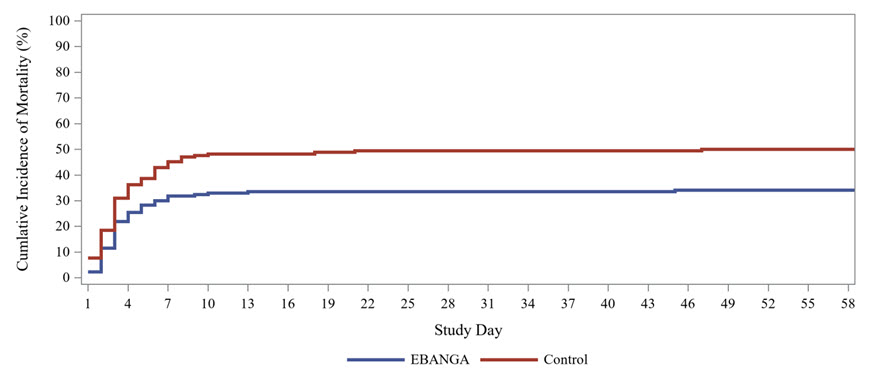
-
16 HOW SUPPLIED / STORAGE AND HANDLING
How Supplied
EBANGA (ansuvimab-zykl) for injection is supplied as a sterile, preservative-free, off-white to white lyophilized powder in a single-dose vial (NDC 80673-001-01) for reconstitution and further dilution.
One primary carton (NDC 80673-001-36) contains thirty-six 400 mg vials packaged in a box containing either one primary carton (NDC 80673-777-01), four primary cartons (NDC 80673-777-04), or eight primary cartons (NDC 80673-777-08).
Storage and Handling
Store refrigerated at 2ºC to 8ºC (36ºF to 46ºF) in the original carton to protect from light. Do not freeze. Do not shake.
Prior to reconstitution, allow EBANGA vial(s) to reach ambient temperature (15°C to 27°C [59°F to 81°F]) for approximately 20 minutes. If for any reason reconstitution cannot proceed immediately upon reaching ambient temperature, vials that have NOT been reconstituted may be kept at ambient temperature, protected from light, for no more than 24 hours.
After reconstitution, if storage is needed, the entire storage time for the reconstituted solution in the vial and the diluted solution in the IV bag should be protected from light and limited to 4 hours refrigerated at 2°C to 8°C (36°F to 46°F) [see Dosage and Administration (2.2)] .
The expiration date for the product is available via a product-specific website with frequent update. The Quick Response (QR) Code at the end of this leaflet is scannable via mobile phone and will direct the user to the EBANGA website. Users will be asked to register for authorized access to lot- specific information via the Lot No. printed on the box containing one, four, or eight 36-vial carton(s). Please access the website using the QR code provided. Alternatively, please go to: www.EBANGA.com. Do not use EBANGA beyond the expiration date available via this website.
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions Including Infusion-Associated Events
Inform patients that hypersensitivity reactions including infusion-associated events have been reported during and post-infusion with EBANGA and to immediately report if they experience any symptoms of systemic hypersensitivity reactions [see Warnings and Precautions (5.1)].
Lactation
Instruct patients with Zaire ebolavirus not to breastfeed because of the risk of passing Zaire ebolavirus to the baby [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 80673-777-01
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 80673-777-04
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 80673-777-08
- PRINCIPAL DISPLAY PANEL - 400 mg Vial Label
- PRINCIPAL DISPLAY PANEL - 400 mg Vial Carton
-
INGREDIENTS AND APPEARANCE
EBANGA
ansuvimab kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80673-777 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80673-777-01 1 in 1 BOX 12/21/2020 1 1 in 1 CARTON 2 NDC:80673-777-04 4 in 1 BOX 12/21/2020 2 1 in 1 CARTON 3 NDC:80673-777-08 8 in 1 BOX 12/21/2020 3 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 36 VIAL, SINGLE-DOSE 288 mL Part 1 of 1 EBANGA
ansuvimab injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:80673-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANSUVIMAB (UNII: TG8IQ19NG2) (ANSUVIMAB - UNII:TG8IQ19NG2) ANSUVIMAB 400 mg in 8 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 12.4 mg in 8 mL HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80673-001-36 36 in 1 CARTON 1 NDC:80673-001-01 8 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761172 12/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761172 12/21/2020 Labeler - Ridgeback Biotherapeutics, LP (116921498)