Label: COMPAZINE- prochlorperazine suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 66213-200-12 - Packager: PBM Pharmaceuticals, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 28, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related PsychosisElderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, com- pared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Compazine ® Prochlorperazine Suppositories USP is not approved for the treatment of patients with dementia-related psychosis (see Warnings).
-
DESCRIPTION
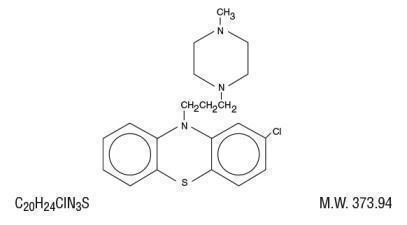
Prochlorperazine, a phenothiazine derivative, is designated chemically as 2-Chloro -10- [3-(4-methyl-1-piperazinyl)propyl]phenothiazine with the following structural formula:
Each suppository, for rectal administration, contains 25 mg of prochlorperazine; with glycerin, glyceryl monopalmitate, glyceryl monostearate, hydrogenated coconut oil fatty acids and hydrogenated palm kernel oil fatty acids.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, narcotics, etc.).
Do not use in pediatric surgery.
Do not use in children under 2 years of age or under 20 lbs. Do not use in children for conditions for which dosage has not been established. -
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Compazine® Prochlorperazine Suppositories USP is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
The extrapyramidal symptoms which can occur secondary to prochlorperazine may be confused with the central nervous system signs of an undiagnosed primary disease responsible for the vomiting, e.g., Reye’s Syndrome or other encephalopathy. The use of prochlorperazine and other potential hepatotoxins should be avoided in children and adolescents whose signs and symptoms suggest Reye’s Syndrome.
Tardive Dyskinesia: Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with neuroleptic (antipsychotic) drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of neuroleptic treatment, which patients are likely to develop the syndrome. Whether neuroleptic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of neuroleptic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if neuroleptic treatment is withdrawn. Neuroleptic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process.
The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, neuroleptics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic neuroleptic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to neuroleptic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on neuroleptics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
For further information about the description of tardive dyskinesia and its clinical detection, please refer to the sections on PRECAUTIONS and ADVERSE REACTIONS.
Neuroleptic Malignant Syndrome (NMS): A potentially fatal symptom complex some- times referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inad- equately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
General: Patients with bone marrow depression or who have previously demonstrated a hypersensitivity reaction (e.g., blood dyscrasias, jaundice) with a phenothiazine should not receive any phenothiazine, including prochlorperazine, unless in the judgment of the physician the potential benefits of treatment outweigh the possible hazards.
Prochlorperazine may impair mental and/or physical abilities, especially during the first few days of therapy. Therefore, caution patients about activities requiring alertness (e.g., operating vehicles or machinery).
Phenothiazines may intensify or prolong the action of central nervous system depressants (e.g., alcohol, anesthetics, narcotics).Usage in Pregnancy: Safety for the use of prochlorperazine during pregnancy has not been established. Therefore, prochlorperazine is not recommended for use in pregnant patients except in cases of severe nausea and vomiting that are so serious and intractable that, in the judgment of the physician, drug intervention is required and potential benefits outweigh possible hazards.
There have been reported instances of prolonged jaundice, extrapyramidal signs, hyperreflexia or hyporeflexia in newborn infants whose mothers received phenothiazines.
Nursing Mothers: There is evidence that phenothiazines are excreted in the breast milk of nursing mothers. -
PRECAUTIONS
Leukopenia, Neutropenia and Agranulocytosis
In clinical trial and postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents.
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a pre- existing low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy
and should discontinue. Compazine® at the first sign of a decline in WBC in the absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with
severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue Compazine® and have their WBC followed until recovery.The antiemetic action of prochlorperazine may mask the signs and symptoms of over- dosage of other drugs and may obscure the diagnosis and treatment of other conditions such as intestinal obstruction, brain tumor and Reye’s Syndrome (see WARNINGS).
When prochlorperazine is used with cancer chemotherapeutic drugs, vomiting as a sign of the toxicity of these agents may be obscured by the antiemetic effect of prochlorperazine.
Because hypotension may occur, large doses and parenteral administration should be used cautiously in patients with impaired cardiovascular systems. If hypotension occurs after parenteral or oral dosing, place patient in head-low position with legs raised. If a vasoconstrictor is required, norepinephrine bitartrate and phenylephrine hydrochloride are suitable. Other pressor agents, including epinephrine, should not be used because they may cause a paradoxical further lowering of blood pressure.
Aspiration of vomitus has occurred in a few post-surgical patients who have received prochlorperazine as an antiemetic. Although no causal relationship has been established, this possibility should be borne in mind during surgical aftercare.
Deep sleep, from which patients can be aroused, and coma have been reported, usually with overdosage.
Neuroleptic drugs elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescribing of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia and impotence have been reported, the clinical significance of elevated serum prolactin lev- els is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of neuroleptic drugs. Neither clinical nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is considered too limited to be conclusive at this time.
Chromosomal aberrations in spermatocytes and abnormal sperm have been demonstrated in rodents treated with certain neuroleptics.
As with all drugs which exert an anticholinergic effect, and/or cause mydriasis, prochlorperazine should be used with caution in patients with glaucoma.
Because phenothiazines may interfere with thermoregulatory mechanisms, use with caution in persons who will be exposed to extreme heat.
Phenothiazines can diminish the effect of oral anticoagulants. Phenothiazines can produce alpha-adrenergic blockade.
Thiazide diuretics may accentuate the orthostatic hypotension that may occur with phenothiazines.
Antihypertensive effects of guanethidine and related compounds may be counteracted when phenothiazines are used concomitantly.
Concomitant administration of propranolol with phenothiazines results in increased plasma levels of both drugs.
Phenothiazines may lower the convulsive threshold; dosage adjustments of anticonvulsants may be necessary. Potentiation of anticonvulsant effects does not occur. However, it has been reported that phenothiazines may interfere with the metabolism of phenytoin and thus precipitate phenytoin toxicity.
The presence of phenothiazines may produce false-positive phenylketonuria (PKU) test results.Long-Term Therapy: Given the likelihood that some patients exposed chronically to neuroleptics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
To lessen the likelihood of adverse reactions related to cumulative drug effect, patients with a history of long-term therapy with prochlorperazine and/or other neuroleptics should be evaluated periodically to decide whether the maintenance dosage could be lowered or drug therapy discontinued.
Children with acute illnesses (e.g., chicken pox, CNS infections, measles, gastro- enteritis) or dehydration seem to be much more susceptible to neuromuscular reactions, particularly dystonias, than are adults. In such patients, the drug should be used only under close supervision.
Drugs which lower the seizure threshold, including phenothiazine derivatives, should not be used with metrizamide. As with other phenothiazine derivatives, prochlorperazine should be discontinued at least 48 hours before myelography, should not be resumed for at least 24 hours postprocedure, and should not be used for the control of nausea and vomiting occurring either prior to myelography with metrizamide, or postprocedure.
Geriatric Use: Clinical studies of prochlorperazine did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. Geriatric patients are more sensitive to the side effects of antipsychotics, including prochlorperazine. These adverse events include hypotension, anticholinergic effects (such as urinary retention, constipation, and confusion), and neuromuscular reactions (such as parkinsonism and tardive dyskinesia) (see PRECAUTIONS and ADVERSE REACTIONS). Also, postmarketing safety experience suggests that the incidence of agranulocytosis may be higher in geriatric patients compared to younger individuals who received prochlorperazine. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see DOSAGE AND ADMINISTRATION).Pregnancy:
Non-teratogenic Effects
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these infants. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases infants have required intensive care unit support and prolonged hospitalization.
Prochlorperazine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. -
ADVERSE REACTIONS
Drowsiness, dizziness, amenorrhea, blurred vision, skin reactions and hypotension may occur.
Cholestatic jaundice has occurred. If fever with grippe-like symptoms occurs, appropriate liver studies should be conducted. If tests indicate an abnormality, stop treatment. There have been a few observations of fatty changes in the livers of patients who have died while receiving the drug. No causal relationship has been established.
Leukopenia and agranulocytosis have occurred. Warn patients to report the sudden appearance of sore throat or other signs of infection. If white blood cell and differential counts indicate leukocyte depression, stop treatment and start antibiotic and other suit- able therapy.
Neuromuscular (Extrapyramidal) Reactions
These symptoms are seen in a significant number of hospitalized mental patients. They may be characterized by motor restlessness, be of the dystonic type, or they may resemble parkinsonism.
Depending on the severity of symptoms, dosage should be reduced or discontinued. If therapy is reinstituted, it should be at a lower dosage. Should these symptoms occur in children or pregnant patients, the drug should be stopped and not reinstituted. In most cases barbiturates by suitable route of administration will suffice. (Or, injectable diphenhydramine may be useful.) In more severe cases, the administration of an anti-parkinsonism agent, except levodopa (see PDR), usually produces rapid reversal of symptoms. Suitable supportive measures such as maintaining a clear airway and adequate hydration should be employed.
Motor Restlessness: Symptoms may include agitation or jitteriness and sometimes insomnia. These symptoms often disappear spontaneously. At times these symptoms may be similar to the original neurotic or psychotic symptoms. Dosage should not be increased until these side effects have subsided.
If these symptoms become too troublesome, they can usually be controlled by a reduction of dosage or change of drug. Treatment with anti-parkinsonian agents, benzodiazepines or propranolol may be helpful.
Dystonia: Class effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment.
Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Pseudo-parkinsonism: Symptoms may include: mask-like facies; drooling; tremors; pillrolling motion; cogwheel rigidity; and shuffling gait. Reassurance and sedation are important. In most cases these symptoms are readily controlled when an anti-parkinson- ism agent is administered concomitantly. Anti-parkinsonism agents should be used only when required. Generally, therapy of a few weeks to 2 or 3 months will suffice. After this time patients should be evaluated to determine their need for continued treatment. (Note: Levodopa has not been found effective in pseudo-parkinsonism.) Occasionally it is nec- essary to lower the dosage of prochlorperazine or to discontinue the drug.
Tardive Dyskinesia: As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy or may appear after drug therapy has been discontinued. The syndrome can also develop, although much less frequently, after relatively brief treatment periods at low doses. This syndrome appears in all age groups. Although its prevalence appears to be highest among elderly patients, especially elderly women, it is impossible to rely upon prevalence estimates to predict at the inception of neuroleptic treatment which patients are likely to develop the syndrome. The symptoms are persis- tent and in some patients appear to be irreversible. The syndrome is characterized by rhythmical involuntary movements of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movements of extremities. In rare instances, these involuntary movements of the extremities are the only manifestations of tardive dyskinesia. A variant of tardive dyskinesia, tardive dystonia, has also been described.
There is no known effective treatment for tardive dyskinesia; anti-parkinsonism agents do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear.
Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked.
It has been reported that fine vermicular movements of the tongue may be an early sign of the syndrome and if the medication is stopped at that time the syndrome may not develop.
Adverse Reactions Reported with Prochlorperazine or Other Phenothiazine Derivatives: Adverse reactions with different phenothiazines vary in type, frequency and mechanism of occurrence, i.e., some are dose-related, while others involve individual patient sensitivity. Some adverse reactions may be more likely to occur, or occur with greater intensity, in patients with special medical problems, e.g., patients with mitral insufficiency or pheochromocytoma have experienced severe hypotension following recommended doses of certain phenothiazines.
Not all of the following adverse reactions have been observed with every phenothiazine derivative, but they have been reported with 1 or more and should be borne in mind when drugs of this class are administered: extrapyramidal symptoms (opisthotonos, oculogyric crisis, hyperreflexia, dystonia, akathisia, dyskinesia, parkinsonism) some of which have lasted months and even years- particularly in elderly patients with previous brain damage; grand mal and petit mal convulsions, particularly in patients with EEG abnormalities or history of such disorders; altered cerebrospinal fluid proteins; cerebral edema; intensification and prolongation of the action of central nervous system depressants (opiates, analgesics, antihistamines, barbiturates, alcohol), atropine, heat, organophosphorus insecticides; autonomic reactions (dryness of the mouth, nasal congestion, headache, nausea, constipation, obstipation, adynamic ileus, ejaculatory disorders/impotence, priapism, atonic colon, urinary retention, miosis and mydriasis); reactivation of psychotic processes, catatonic-like states; hypotension (sometimes fatal); cardiac arrest; blood dyscrasias (pancytopenia, thrombocytopenic purpura, leukopenia, agranulocytosis, eosinophilia, hemolytic anemia, aplastic anemia); liver damage (jaundice, biliary stasis); endocrine disturbances (hyperglycemia, hypoglycemia, glycosuria, lactation, galactorrhea, gynecomastia, menstrual irregularities, false-positive pregnancy tests); skin disorders (photosensitivity, itching, erythema, urticaria, eczema up to exfoliative dermatitis); other allergic reactions (asthma, laryngeal edema, angioneurotic edema, anaphylactoid reactions); peripheral edema; reversed epinephrine effect; hyperpyrexia; mild fever after large I.M. doses; increased appetite; increased weight; a systemic lupus erythematosus-like syndrome; pigmentary retinopathy; with prolonged administration of substantial doses, skin pigmentation, epithelial keratopathy, and lenticular and corneal deposits.
EKG changes-particularly nonspecific, usually reversible Q and T wave distortions-have been observed in some patients receiving phenothiazine tranquilizers.
Although phenothiazines cause neither psychic nor physical dependence, sudden dis- continuance in long-term psychiatric patients may cause temporary symptoms, e.g., nau- sea and vomiting, dizziness, tremulousness.
Note: There have been occasional reports of sudden death in patients receiving phenothiazines. In some cases, the cause appeared to be cardiac arrest or asphyxia due to failure of the cough reflex.
To Report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-800-328-5113, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -
OVERDOSAGE
(See also ADVERSE REACTIONS).
SYMPTOMS–Primarily involvement of the extrapyramidal mechanism producing some of the dystonic reactions described above.
Symptoms of central nervous system depression to the point of somnolence or coma. Agitation and restlessness may also occur. Other possible manifestations include convulsions, EKG changes and cardiac arrhythmias, fever and autonomic reactions such as hypotension, dry mouth and ileus.
TREATMENT–It is important to determine other medications taken by the patient since multiple-dose therapy is common in overdosage situations. Treatment is essentially symptomatic and supportive. Early gastric lavage is helpful. Keep patient under observation and maintain an open airway, since involvement of the extrapyramidal mechanism may produce dysphagia and respiratory difficulty in severe overdosage. Do not attempt to induce emesis because a dystonic reaction of the head or neck may develop that could result in aspiration of vomitus. Extrapyramidal symptoms may be treated with anti-parkinsonism drugs, barbiturates or diphenhydramine. See prescribing information for these products. Care should be taken to avoid increasing respiratory depression.
If administration of a stimulant is desirable, amphetamine, dextroamphetamine or caffeine with sodium benzoate is recommended.
Stimulants that may cause convulsions (e.g., picrotoxin or pentylenetetrazol) should be avoided.
If hypotension occurs, the standard measures for managing circulatory shock should be initiated. If it is desirable to administer a vasoconstrictor, norepinephrine bitartrate and phenylephrine hydrochloride are most suitable. Other pressor agents, including epineph- rine, are not recommended because phenothiazine derivatives may reverse the usual elevating action of these agents and cause a further lowering of blood pressure.
Limited experience indicates that phenothiazines are not dialyzable. -
DOSAGE AND ADMINISTRATION
Adults: Dosage should be increased more gradually in debilitated or emaciated patients.
Elderly Patients: In general, dosages in the lower range are sufficient for most elderly patients. Since they appear to be more susceptible to hypotension and neuromuscular reactions, such patients should be observed closely. Dosage should be tailored to the individual, response carefully monitored and dosage adjusted accordingly. Dosage should be increased more gradually in elderly patients.
To Control Severe Nausea and Vomiting: Adjust dosage to the response of the individual. Begin with the lowest recommended dosage.
Rectal Dosage: 25 mg twice daily. -
HOW SUPPLIED
Compazine® Prochlorperazine Suppositories USP, 25mg (for adults) are easy to open, and available in boxes of 12.
12’s – NDC 66213-200-12
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Do not remove from wrapper until ready to use.Manufactured for PBM Pharmaceuticals, Inc.
Charlottesville, VA 22902
2202921 Rev 04-13 A -
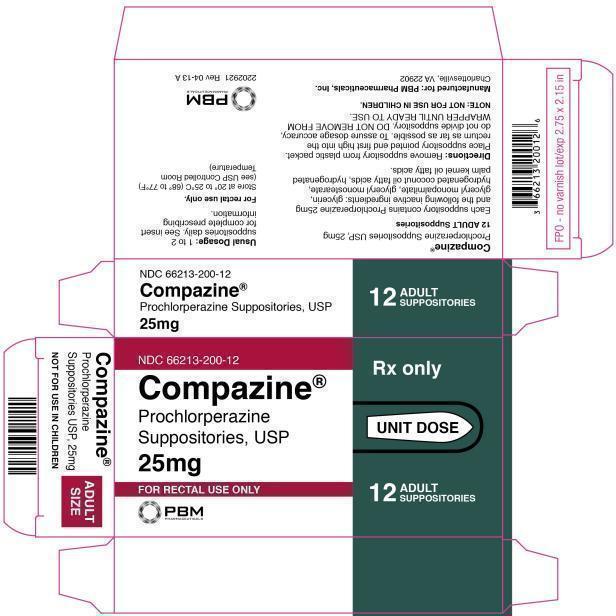
Compazine Carton Label
NDC 66213-200-12
Compazine®
Prochlorperazine
Suppositories, USP
25mg
FOR RECTAL USE ONLYRX ONLY
UNIT DOSE
12
ADULT
SUPPOSITORIES
COMPAZINE®
Prochlorperazine
Suppositories USP, 25mg
NOT FOR USE IN CHILDREN
ADULT
SIZE
NDC 66213-200-12
Compazine®
Prochlorperazine Suppositories, USP
25mg
COMPAZINE®
Prochlorperazine Suppositories USP, 25mg
12 ADULT Suppositories
Each suppository contains Prochlorperazine 25mg and the following inactive ingredients: glycerin, glyceryl monopalmitate, glyceryl monostearate, hydrogenated coconut oil fatty acids, hydrogenated palm kernel oil fatty acids.
Directions: Remove suppository from plastic packet. Place suppository pointed end first high into the rectum as far as possible. To assure dosage accuracy, do not divide suppository. DO NOT REMOVE FROM WRAPPER UNTIL READY TO USE.
NOTE: NOT FOR USE IN CHILDREN.
Manufactured for: PBM Pharmaceuticals, Inc.
Charlottesville, VA 22902
USUAL DOSAGE: 1 TO 2 SUPPOSITORIES DAILY. SEE INSERT FOR COMPLETE PRESCRIBING INFORMATION.
For rectal use only.
Store at 20° to 25°C (68° to 77°F)
(see USP Controlled Room Temperature)
2202921 REV 04-13 A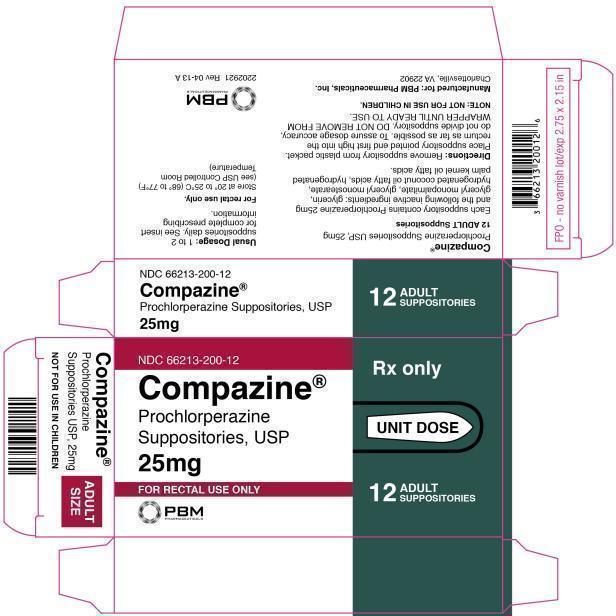
-
INGREDIENTS AND APPEARANCE
COMPAZINE
prochlorperazine suppositoryProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66213-200 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Prochlorperazine (UNII: YHP6YLT61T) (PROCHLORPERAZINE - UNII:YHP6YLT61T) Prochlorperazine 25 mg Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Glyceryl Palmitate (UNII: 6Y2XJ05B35) Glyceryl Monostearate (UNII: 230OU9XXE4) Hydrogenated Coconut Oil (UNII: JY81OXM1OM) PALM KERNEL OIL (UNII: B0S90M0233) Product Characteristics Color white (White to Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66213-200-12 12 in 1 CARTON 1 1 in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040246 07/01/2013 Labeler - PBM Pharmaceuticals, Inc (785470050)