SODIUM CHLORIDE- sodium chloride injection
B. Braun Medical Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sodium Chloride 0.9% w/v Intravenous Infusion
IMPORTANT PRESCRIBING INFORMATION
B. Braun Medical Inc.
824 12th Avenue
Bethlehem, PA 18018
610-691-5400
October 20, 2017
Subject: Temporary Importation of 0.9% Sodium Chloride w/v Intravenous Infusion in Ecoflac Plus Containers
Dear Health Care Provider,
The purpose of this letter is to inform you of an additional foreign product that B. Braun Medical Inc. (B. Braun) will be providing in the United States (U.S.) to address the critical drug shortage of 0.9% Sodium Chloride for Injection. Due to the shortage of 0.9% Sodium Chloride Injection products in the U.S., B. Braun is coordinating with the U.S. Food and Drug Administration (FDA) to temporarily import foreign Sodium Chloride 0.9% w/v Intravenous Infusion BP in the Ecoflac plus container into the U.S. market. The foreign product is manufactured at an FDA inspected B. Braun sterile injectable facility in Melsungen, Germany which is currently in compliance with FDA regulations.
At this time, no other entity except B. Braun is authorized by the FDA to import or directly/indirectly distribute Sodium Chloride 0.9% w/v Intravenous Infusion BP in the Ecoflac® plus container in the U.S. FDA has not approved B. Braun’s Sodium Chloride 0.9% w/v Intravenous Infusion BP in the Ecoflac plus container in the United States.
Effective immediately and during this temporary period, B. Braun will offer the following presentations of B. Braun’s Sodium Chloride 0.9% w/v Intravenous Infusion BP in the Ecoflac plus container:
| Product Name | Volume | Ingredients |
| Sodium Chloride 0.9% w/v Intravenous Infusion BP in the Ecoflac plus container | 1,000 mL | Each 1,000 mL contains: Sodium Chloride 9.0 g, Water for Injections to 1,000 mL Electrolytes per 1,000 mL is Sodium 154 mmol, Chloride 154 mmol |
Ecoflac plus container- Not made with natural rubber latex, PVC, or DEHP.
Indications and Usage and Dosage Administration
The foreign unapproved product, packaged in a semi-rigid Ecoflac® (polyethylene plastic) container, contains the same active ingredient in the same concentration as the 0.9% Sodium Chloride Injection products approved in the U. S., packaged in the flexible plastic EXCEL® (ethylene-propylene copolymer) bag. As such, clinical practice pertaining to indication, usage and dosage administration for Sodium Chloride 0.9% w/v Intravenous Infusion BP in the Ecoflac® plus container is the same as with the EXCEL® containers.
However, some key differences between the Ecoflac and EXCEL® container packaging and labeling are described below in the Product Comparison Table.
It is also important to note that the Ecoflac plus container is a semi rigid plastic bottle. However, no venting is necessary during infusion. Ecoflac® plus collapses completely when emptying.
Ecoflac plus container and carton labeling may include barcodes that may not register accurately in the U.S. scanning systems. Institutions should manually input the product into their systems and confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
Reporting Adverse Events
To report adverse events or quality problems with B. Braun’s Sodium Chloride 0.9% w/v Intravenous Infusion BP in the Ecoflac® plus container, please contact the B. Braun Clinical and Technical Support Department at 1-800-854-6851. Adverse events that may be related to the use of this product may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, or regular mail or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Thank you for your commitment to B. Braun’s IV solution products.
Tom Sutton
Vice President of Marketing
| PRODUCT COMPARISON TABLE | ||
| Item | German Product – Ecoflac® Container | US Product - EXCEL® Container |
| Product Drawing |
Ecoflac® Plus 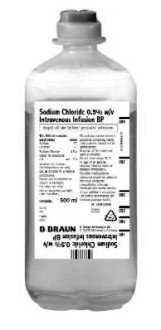 |
EXCEL®  |
| Product Description | 0.9% w/v Sodium Chloride BP | 0.9% Sodium Chloride Injection USP |
| Product Code | 9999-00 | L8000 |
| Unit Bar Code | No | Yes (NDC and Lot/Exp) |
| NDC # | 0264-9999-00 | 0264-7800-00 |
| Volume | 1,000 mL | 1,000 mL |
| Case Quantity | 10 units per case | 12 units per case |
| Storage Condition | Do not store above 25°C | Store at room temperature (25°C) |
| Shelf Life | 36 months | 30 months |
| Ingredients | Each 1,000 mL contains: Sodium Chloride EP 900 mg, Water for Injection EP to 1,000 mL Total Electrolytes per 1,000 mL : Sodium 154 mEq, Chloride 154 mEq | Each 1,000 mL contains: Sodium Chloride USP 900 mg, Water for Injection USP to 1,000 mL. Total Electrolytes per 1,000 mL: Sodium 154 mEq, Chloride 154 mEq |
| Container Type | Ecoflac® Plus, Polyethylene Plastic Containers (semi rigid plastic bottle) | Excel®, Primary plastic container with a clear overwrap (flexible plastic bag) |
| Container material | Low Density Polyethylene (LDPE) | Copolymer of ethylene and propylene |
| Container Description | Blow/Fill/Seal (BFS) | Form/Fill/Seal (FFS) |
| PVC, DEHP, Latex | No | No |
| Overwrap | No | Yes |
| Closure Description | Twin cap with peel tab cover | Additive port with elastomeric stopper, Administration port with plastic cover |
| Pictures of Difference in Container Port System |
Twin Port with peel tab cover and two interchangeable access ports with thermoplastic elastomeric septum  |
Separate additive and administration ports 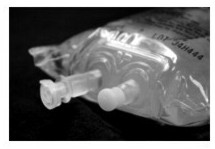 |
| Additive Port Material | Thermoplastic elastomer | Synthetic Isoprene |
| Needle Size | 18-21 gauge | 18-22 gauge |
| Additive Volume | 170 mL | 200 mL |
| Spike Port Resealable | Yes | No |
| Pressure Infusion | No | Yes, not to exceed 300mm Hg |
| Sterilization Process | Terminal Steam Sterilization Sterility Test (Ph. Eur.) Sterility Assurance Level of 10-6 | Terminal Steam Sterilization Parametric Release Sterility Assurance Level of 10-6 |
PRINCIPAL DISPLAY PANEL
Sodium Chloride 0.9% w/v
Intravenous Infusion BP
Expel all air before pressure infusion.
This 1000 ml contains:
Electrolytes mmol
Sodium 154
Chloride 154
Sodium Chloride 9.0 g
Water for injections
pH 4.5 - 7.0
Solution for infusion. For intravenous use only. Use as directed by a medical practitioner. Do not use if container or closure is damaged. For single use only. Do not reconnect partially used containers.
Contents: 1000 ml
Discard any unused contents. Solutions containing visible solid particles should not be administered. Keep out of the reach and sight of children. Do not store above 25 °C. In case of an adverse reaction, infusion must be stopped immediately. No other medication or substance should be added to this fluid unless the compatibility is known. Thorough and careful mixing of any additive is mandatory. For further information, see enclosed package leaflet.
LOT
EXP:

B. Braun Melsungen AG
D-34209 Melsungen
Germany
5/12625007/1117

| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - B. Braun Medical Inc. (002397347) |