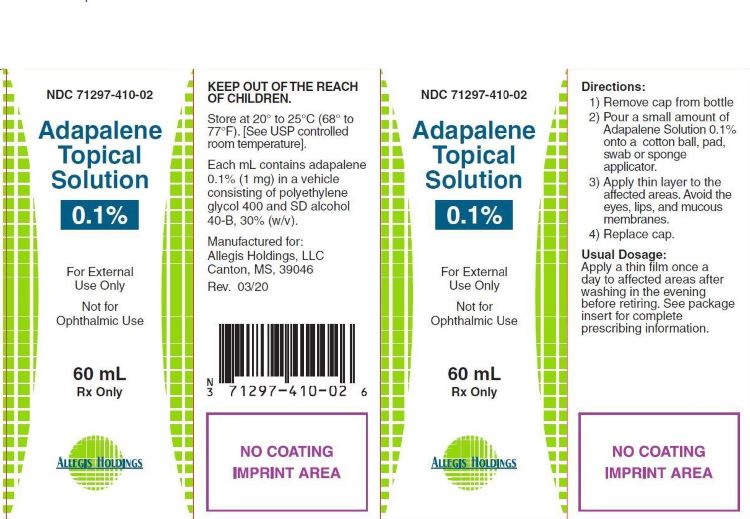
Label: ADAPALENE TOPICAL SOLUTION solution
- NDC Code(s): 71297-410-02
- Packager: Allegis Holdings LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Adapalene Topical Solution 0.1%, containing adapalene is used for the topical treatment of acne vulgaris. Each mL of Adapalene Topical Solution, 0.1%, contains adapalene 0.1% (1 mg) in a vehicle consisting of polyethylene glycol 400 and alcohol, denatured, 30% (w/v).
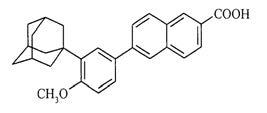
The chemical name of adapalene is 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid. Adapalene is a white to off-white powder which is soluble in tetrahydrofuran, sparingly soluble in ethanol, and practically insoluble in water. The molecular formula is C 28H 28O 3 and molecular weight is 412.52. Adapalene is represented by the following structural formula:
-
CLINICAL PHARMACOLOGY:
Adapalene is a chemically stable, retinoid-like compound. Biochemical and pharmacological profile studies have demonstrated that adapalene is a modulator of cellular differentiation, keratinization, and inflammatory processes all of which represent important features in the pathology of acne vulgaris. Mechanistically, adapalene binds to specific retinoic acid nuclear receptors but does not bind to the cytosolic receptor protein. Although the exact mode of action is unknown, it is suggested that topical adapalene may normalize the differentiation of follicular epithelial cells resulting in decreased microcomedone formation.
Pharmacokinetics:
Absorption of adapalene through human skin is low. Only trace amounts (< 0.25 ng/mL) of parent substance have been found in the plasma of acne patients following chronic topical application of adapalene in controlled clinical trials. Excretion appears to be primarily by the biliary route.
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
General:
If a reaction suggesting sensitivity or chemical irritation occurs, use of the medication should be discontinued. Exposure to sunlight, including sunlamps, should be minimized during the use of adapalene. Patients who normally experience high levels of sun exposure, and those with inherent sensitivity to sun, should be warned to exercise caution. Use of sunscreen products and protective clothing over treated areas is recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, also may be irritating to patients under treatment with adapalene.
Avoid contact with the eyes, lips, angles of the nose, and mucous membranes. The product should not be applied to cuts, abrasions, eczematous skin, or sunburned skin.
Certain cutaneous signs and symptoms such as erythema, dryness, scaling, burning, or pruritus may be experienced during treatment. These are most likely to occur during the first two to four weeks and will usually lessen with continued use of the medication. Depending upon the severity of adverse events, patients should be instructed to reduce the frequency of application or discontinue use.
Drug Interactions:
As Adapalene Topical Solution 0.1% has the potential to produce local irritation in some patients, concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect, and products with high concentrations of alcohols, astringents, spices or lime) should be approached with caution. Particular caution should be exercised in using preparations containing sulfur, resorcinol, or salicylic acid in combination with Adapalene Topical Solution 0.1%. If these preparations have been used, it is advisable not to start therapy with Adapalene Topical Solution 0.1% until the effects of such preparations in the skin have subsided.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenicity studies with adapalene have been conducted in mice at topical doses of 0.3, 0.9, and 2.6 mg/kg/day and in rats at oral doses of 0.15, 0.5, and 1.5 mg/kg/day, approximately 4-75 times the maximal daily human topical dose. In the oral study, positive linear trends were observed in the incidence of follicular cell adenomas and carcinomas in the thyroid glands of female rats, and in the incidence of benign and malignant pheochromocytomas in the adrenal medullas of male rats.
No photocarcinogenicity studies were conducted. Animal studies have shown an increased tumorigenic risk with the use of pharmacologically similar drugs (e.g., retinoids) when exposed to UV irradiation in the laboratory or to sunlight. Although the significance of these studies to human use is not clear, patients should be advised to avoid or minimize exposure to either sunlight or artificial UV irradiation sources.
In a series of in vivo and in vitro studies, adapalene did not exhibit mutagenic or genotoxic activities.
Pregnancy: Teratogenic effects. Pregnancy Category C.
No teratogenic effects were seen in rats at oral doses of adapalene 0.15 to 5.0 mg/kg/day, up to 120 times the maximal daily human topical dose. Cutaneous route teratology studies conducted in rats and rabbits at doses of 0.6, 2.0, and 6.0 mg/kg/day, up to 150 times the maximal daily human topical dose exhibited no fetotoxicity and only minimal increases in supernumerary ribs in rats. There are no adequate well-controlled studies in pregnant women. Adapalene should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
ADVERSE REACTIONS:
Some adverse effects such as erythema, scaling, dryness, pruritus, and burning will occur in 30-60% of patients. Pruritus or burning immediately after application also occurs in approximately 30% of patients. The following additional adverse experiences were reported in approximately 1% or less of patients: skin irritation, burning/stinging, erythema, sunburn, and acne flares. These are most commonly seen during the first month of therapy and decrease in frequency and severity thereafter. All adverse effects with the use of similar products during clinical trials were reversible upon discontinuation of therapy.
To report SUSPECTED ADVERSE REACTIONS, contact Allegis Holding, LLC at 1-866-633-9033 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE:
Adapalene Topical Solution 0.1% is intended for cutaneous use only. If the medication is applied excessively, no more rapid or better results will be obtained and marked redness, peeling, or discomfort may occur. The acute oral toxicity of Adapalene Topical Solution 0.1% in mice and rats is greater than 10 mL/kg. Chronic ingestion of the drug may lead to the same side effects as those associated with excessive oral intake of Vitamin A.
-
DOSAGE AND ADMINISTRATION:
1. Adapalene Topical Solution 0.1% should be applied once a day to affected areas.
2. Before retiring in the evening, wash and dry areas to be treated.
3. Apply a thin film of medication to the affected areas. Avoid the eyes lips, and mucous membranes.
4. Replace cap after each use.During the early weeks of therapy, an apparent exacerbation of acne may occur. This is due to the action of the medication on previously unseen lesions and should not be considered a reason to discontinue therapy. Therapeutic results should be noticed after eight to twelve weeks of treatment.
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ADAPALENE TOPICAL SOLUTION
adapalene topical solution solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71297-410 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADAPALENE (UNII: 1L4806J2QF) (ADAPALENE - UNII:1L4806J2QF) ADAPALENE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71297-410-02 1 in 1 CARTON 10/03/2016 1 60 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203981 10/03/2016 Labeler - Allegis Holdings LLC (080556861)