MIRENA- levonorgestrel intrauterine device
Bayer HealthCare Pharmaceuticals Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Mirena safely and effectively. See full prescribing information for Mirena.
Mirena (levonorgestrel-releasing intrauterine system) Initial U.S. Approval: 2000 RECENT MAJOR CHANGES
INDICATIONS AND USAGEMirena is a sterile, levonorgestrel-releasing intrauterine system indicated for:
It is recommended for women who have had at least one child. DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSOne sterile intrauterine system consisting of a T-shaped polyethylene frame with a steroid reservoir containing 52 mg levonorgestrel packaged within a sterile inserter (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions reported in clinical trials (> 10% users) are uterine/vaginal bleeding alterations (51.9%), amenorrhea (23.9%), intermenstrual bleeding and spotting (23.4%), abdominal /pelvic pain (12.8%) and ovarian cysts (12%). (6) To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 11/2017 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
- •
- Mirena is indicated for intrauterine contraception for up to 5 years.
- •
- Mirena is also indicated for the treatment of heavy menstrual bleeding in women who choose to use intrauterine contraception as their method of contraception.
Mirena is recommended for women who have had at least one child.
The system should be replaced after 5 years if continued use is desired.
2 DOSAGE AND ADMINISTRATION
Mirena contains 52 mg of levonorgestrel. Initially, levonorgestrel is released at a rate of approximately 20 mcg/day. This rate decreases progressively to half that value after 5 years.
Mirena is packaged sterile within an inserter. Information regarding insertion instructions, patient counseling and record keeping, patient follow-up, removal of Mirena and continuation of contraception after removal is provided below.
2.1. Insertion Instructions
- •
- NOTE: Mirena should be inserted by a trained healthcare provider. Healthcare providers are advised to become thoroughly familiar with the insertion instructions before attempting insertion of Mirena.
- •
- Mirena is inserted with the provided inserter (Figure 1a) into the uterine cavity within seven days of the onset of menstruation or immediately after a first trimester abortion by carefully following the insertion instructions. It can be replaced by a new Mirena at any time during the menstrual cycle.
Preparation for insertion
- •
- Ensure that the patient understands the contents of the Patient Information Booklet and obtain consent. A consent form that includes the lot number is on the last page of the Patient Information Booklet.
- •
- Confirm that there are no contraindications to the use of Mirena.
- •
- Perform a urine pregnancy test, if indicated.
- •
- With the patient comfortably in lithotomy position, gently insert a speculum to visualize the cervix and rule out genital contraindications to the use of Mirena.
- •
- Do a bimanual exam to establish the size and position of the uterus, to detect other genital contraindications, and to exclude pregnancy.
- •
- Thoroughly cleanse the cervix and vagina with a suitable antiseptic solution. Perform a paracervical block, if needed.
- •
- Prepare to sound the uterine cavity. Grasp the upper lip of the cervix with a tenaculum forceps and apply gentle traction to align the cervical canal with the uterine cavity. If the uterus is retroverted, it may be more appropriate to grasp the lower lip of the cervix. Note that the tenaculum forceps should remain in position throughout the insertion procedure to maintain gentle traction on the cervix.
- •
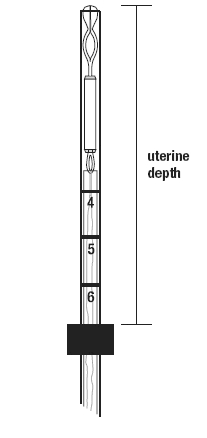
- Gently insert a uterine sound to check the patency of the cervix, measure the depth of the uterine cavity, confirm its direction and exclude the presence of any uterine anomaly. If you encounter cervical stenosis, use dilatation, not force, to overcome resistance.
- •
- The uterus should sound to a depth of 6 to 10 cm. Insertion of Mirena into a uterine cavity less than 6 cm by sounding may increase the incidence of expulsion, bleeding, pain, perforation, and possibly pregnancy.
- •
- After ascertaining that the patient is appropriate for Mirena, open the carton containing Mirena.
Insertion Procedure
Ensure use of sterile technique throughout the entire procedure.
Step 1–Opening of the sterile package
- •
- Open the sterile package completely (Figure 1b).
- •
- Place sterile gloves on your hands.
- •
- Pick up the handle of the inserter containing Mirena and carefully release the threads so that they hang freely.
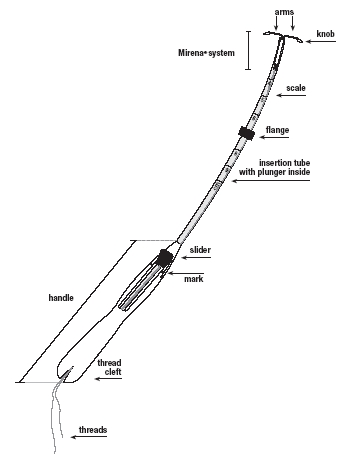
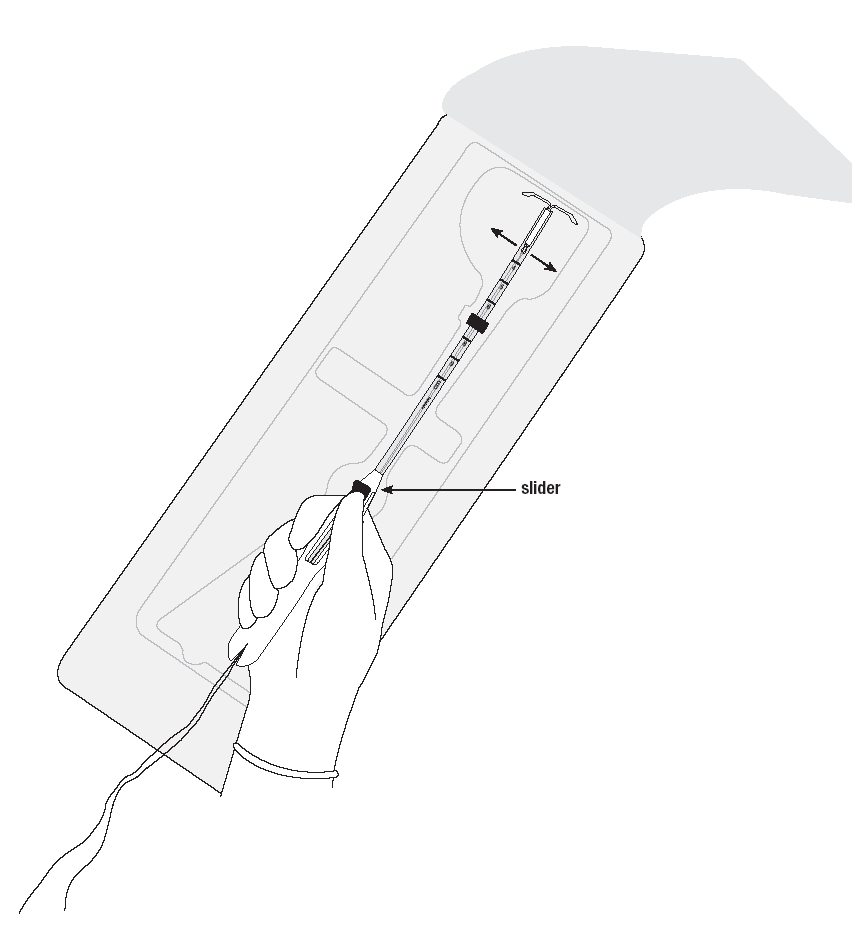
Place your thumb or forefinger on the slider. Make sure that the slider is in the furthest position away from you, for example, at the top of the handle towards the insertion tube (Figure 1b).
NOTE: Keep your thumb or forefinger on the slider until insertion is complete.
Step 2–Load Mirena into the insertion tube
- •
- Holding the slider in the furthest position, pull on both threads to load Mirena into the insertion tube (Figure 2a).
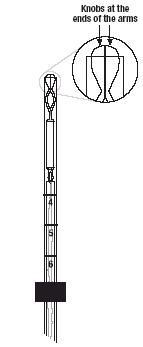
- •
- Note that the knobs at the ends of the arms now meet to close the open end of the insertion tube (Figure 2b).
- If the knobs do not meet properly
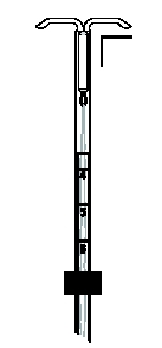
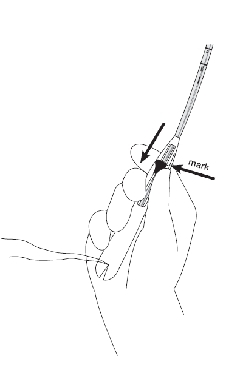
If the knobs do not meet properly, release the arms by pulling the slider back to the mark (raised horizontal line on the handle) (Figure 6a) . Re-load Mirena by aligning the open arms on a sterile surface (Figure 1b). Return the slider to its furthermost position and pull on both threads. Check for proper loading (Figure 2b).
Step 3–Secure the threads
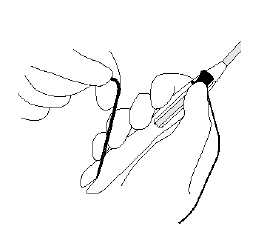
Secure the threads in the cleft at the bottom end of the handle to keep Mirena in the loaded position (Figure 3).
Step 4–Setting the flange
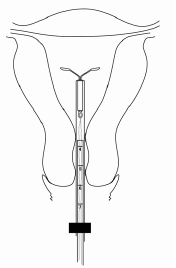
Set the upper edge of the flange to the depth measured during the uterine sounding (Figure 4).
Step 5–Mirena is now ready to be inserted
- •
- Continue to hold the slider with the thumb or forefinger firmly in the furthermost position. Grasp the tenaculum forceps with your other hand and apply gentle traction to align the cervical canal with the uterine cavity.
- •
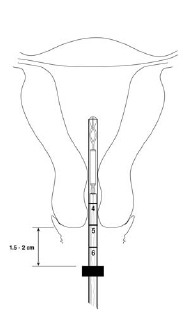
- While maintaining traction on the cervix, gently advance the insertion tube through the cervical canal and into the uterine cavity until the flange is 1.5 to 2 cm from the external cervical os.
- •
- CAUTION: do not advance flange to the cervix at this step. Maintaining the flange 1.5 to 2 cm from the cervical os allows sufficient space for the arms to open (when released) within the uterine cavity (Figures 5 and 6b).
- NOTE! Do not force the inserter. If necessary, dilate the cervical canal.
Step 6–Release the arms
- •
- While holding the inserter steady, release the arms of Mirena by pulling the slider back until the top of the slider reaches the mark (raised horizontal line on the handle) (Figure 6a).
- •
- Wait approximately 10 seconds to allow the horizontal arms of Mirena to open and regain its T-shape (Figure 6b).
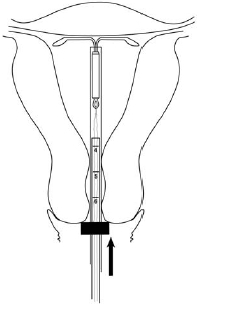
Step 7–Advance to fundal position
Gently advance the inserter into the uterine cavity until the flange meets the cervix and you feel fundal resistance. Mirena should now be in the desired fundal position (Figure 7).
Step 8–Release Mirena and withdraw the inserter
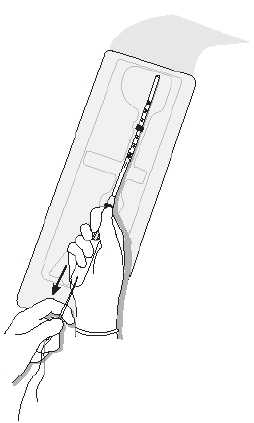
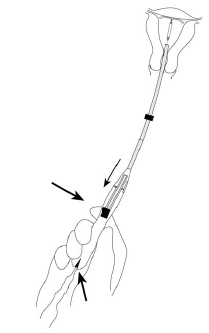
- •
- While holding the inserter steady, pull the slider all the way down to release Mirena from the insertion tube (Figure 8). The threads will release automatically from the cleft.
- •
- Check that the threads are hanging freely and gently withdraw the inserter from the uterus. Be careful not to pull on the threads as this will displace Mirena.
Step 9–Cut the threads
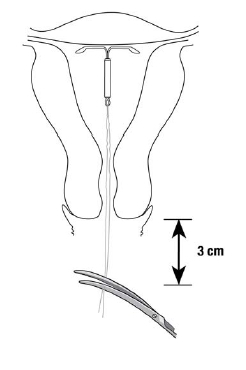
- •
- Cut the threads perpendicular to the thread length, for example, with sterile curved scissors, leaving about 3 cm visible outside the cervix (Figure 9).
NOTE: Cutting threads at an angle may leave sharp ends.
Mirena insertion is now complete.
Important information to consider during or after insertion
- •
- If you suspect that Mirena is not in the correct position, check placement (for example, with transvaginal ultrasound). Remove Mirena if it is not positioned completely within the uterus. A removed Mirena must not be reinserted.
- •
- If there is clinical concern and/or exceptional pain or bleeding during or after insertion, appropriate and timely measures and assessments, for example ultrasound, should be performed to exclude perforation.
2.2 Patient Counseling and Record Keeping
- •
- Keep a copy of the consent form and lot number for your records.
- •
- Counsel the patient on what to expect following Mirena insertion. Give the patient the Follow-up Reminder Card that is provided with the product. Discuss expected bleeding patterns during the first months of Mirena use. [See Patient Counseling Information (17.1).]
- •
- Prescribe analgesics, if indicated.
2.3 Patient Follow-up
- •
- Patients should be reexamined and evaluated 4 to 12 weeks after insertion and once a year thereafter, or more frequently if clinically indicated.
2.4 Removal of Mirena
- •
- Remove Mirena by applying gentle traction on the threads with forceps. The arms will fold upward as it is withdrawn from the uterus. Mirena should not remain in the uterus after 5 years.
- •
- Removal may be associated with some pain and/or bleeding or neurovascular episodes.
- •
- If the threads are not visible and Mirena is in the uterine cavity, it may be removed using a narrow forceps, such as an alligator forceps. This may require dilation of the cervical canal [see Warnings and Precautions (5.13)].
- •
- After removal of Mirena, verify that the system is intact.
- •
- During difficult removals, the hormone cylinder may slide over and cover the horizontal arms. This situation generally does not require further intervention once the system is verified to be intact.
- •
- If Mirena is removed mid-cycle and the woman has had intercourse within the preceding week, she is at a risk of pregnancy unless a new Mirena is inserted immediately following removal.
2.5 Continuation of Contraception after Removal
- •
- You may insert a new Mirena immediately following removal.
- •
- If a patient with regular cycles wants to start a different birth control method, remove Mirena during the first 7 days of the menstrual cycle and start the new method.
- •
- If a patient with irregular cycles or amenorrhea wants to start a different birth control method, or if you remove Mirena after the seventh day of the menstrual cycle, start the new method at least 7 days before removal.
3 DOSAGE FORMS AND STRENGTHS
Mirena is a levonorgestrel-releasing intrauterine system consisting of a T-shaped polyethylene frame with a steroid reservoir containing a total of 52 mg levonorgestrel.
4 CONTRAINDICATIONS
The use of Mirena is contraindicated when one or more of the following conditions exist:
- •
- Pregnancy or suspicion of pregnancy
- •
- Congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity
- •
- Acute pelvic inflammatory disease or a history of pelvic inflammatory disease unless there has been a subsequent intrauterine pregnancy
- •
- Postpartum endometritis or infected abortion in the past 3 months
- •
- Known or suspected uterine or cervical neoplasia or unresolved, abnormal Pap smear
- •
- Genital bleeding of unknown etiology
- •
- Untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infections until infection is controlled
- •
- Acute liver disease or liver tumor (benign or malignant)
- •
- Conditions associated with increased susceptibility to pelvic infections
- •
- A previously inserted IUD that has not been removed
- •
- Hypersensitivity to any component of this product
- •
- Known or suspected carcinoma of the breast.
5 WARNINGS AND PRECAUTIONS
5.1 Ectopic Pregnancy
Evaluate women who become pregnant while using Mirena for ectopic pregnancy. Up to half of pregnancies that occur with Mirena in place are ectopic. The incidence of ectopic pregnancy in clinical trials that excluded women with risk factors for ectopic pregnancy was approximately 0.1% per year.
Tell women who choose Mirena about the risks of ectopic pregnancy, including the loss of fertility. Teach them to recognize and report to their physician promptly any symptoms of ectopic pregnancy. Women with a previous history of ectopic pregnancy, tubal surgery or pelvic infection carry a higher risk of ectopic pregnancy.
The risk of ectopic pregnancy in women who have a history of ectopic pregnancy and use Mirena is unknown. Clinical trials of Mirena excluded women with a history of ectopic pregnancy.
5.2 Intrauterine Pregnancy
If pregnancy should occur with Mirena in place, Mirena should be removed. Removal or manipulation of Mirena may result in pregnancy loss. In the event of an intrauterine pregnancy with Mirena, consider the following:
Septic abortion
In patients becoming pregnant with an IUD in place, septic abortion—with septicemia, septic shock, and death—may occur.
Continuation of pregnancy
If a woman becomes pregnant with Mirena in place and if Mirena cannot be removed or the woman chooses not to have it removed, she should be warned that failure to remove Mirena increases the risk of miscarriage, sepsis, premature labor and premature delivery. She should be followed closely and advised to report immediately any flu-like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge or leakage of fluid.
Long-term effects and congenital anomalies
When pregnancy continues with Mirena in place, long-term effects on the offspring are unknown. As of September 2006, 390 live births out of an estimated 9.9 million Mirena users had been reported. Congenital anomalies in live births have occurred infrequently. No clear trend towards specific anomalies has been observed. Because of the intrauterine administration of levonorgestrel and local exposure of the fetus to the hormone, the possibility of teratogenicity following exposure to Mirena cannot be completely excluded. Some observational data support a small increased risk of masculinization of the external genitalia of the female fetus following exposure to progestins at doses greater than those currently used for oral contraception. Whether these data apply to Mirena is unknown.
5.3 Sepsis
As of September 2006, 9 cases of Group A streptococcal sepsis (GAS) out of an estimated 9.9 million Mirena users had been reported. In some cases, severe pain occurred within hours of insertion followed by sepsis within days. Because death from GAS is more likely if treatment is delayed, it is important to be aware of these rare but serious infections. Aseptic technique during insertion of Mirena is essential. GAS sepsis may also occur postpartum, after surgery, and from wounds.
5.4 Pelvic Inflammatory Disease (PID)
Mirena is contraindicated in the presence of known or suspected PID or in women with a history of PID unless there has been a subsequent intrauterine pregnancy. Use of IUDs has been associated with an increased risk of PID. The highest risk of PID occurs shortly after insertion (usually within the first 20 days thereafter) [see Warnings and Precautions (5.12)]. A decision to use Mirena must include consideration of the risks of PID.
Women at increased risk for PID
PID is often associated with a sexually transmitted disease, and Mirena does not protect against sexually transmitted disease. The risk of PID is greater for women who have multiple sexual partners, and also for women whose sexual partner(s) have multiple sexual partners. Women who have had PID are at increased risk for a recurrence or re-infection.
PID warning to Mirena users
All women who choose Mirena must be informed prior to insertion about the possibility of PID and that PID can cause tubal damage leading to ectopic pregnancy or infertility, or infrequently can necessitate hysterectomy, or cause death. Patients must be taught to recognize and report to their physician promptly any symptoms of pelvic inflammatory disease. These symptoms include development of menstrual disorders (prolonged or heavy bleeding), unusual vaginal discharge, abdominal or pelvic pain or tenderness, dyspareunia, chills, and fever.
Treatment of PID
Following a diagnosis of PID, or suspected PID, bacteriologic specimens should be obtained and antibiotic therapy should be initiated promptly. Removal of Mirena after initiation of antibiotic therapy is usually appropriate. Guidelines for PID treatment are available from the Centers for Disease Control (CDC), Atlanta, Georgia.
Actinomycosis has been associated with IUDs. Symptomatic women with IUDs should have the IUD removed and should receive antibiotics. However, the management of the asymptomatic carrier is controversial because actinomycetes can be found normally in the genital tract cultures in healthy women without IUDs. False positive findings of actinomycosis on Pap smears can be a problem. When possible, confirm the Pap smear diagnosis with cultures.
5.5 Irregular Bleeding and Amenorrhea
Mirena can alter the bleeding pattern and result in spotting, irregular bleeding, heavy bleeding, oligomenorrhea and amenorrhea. During the first three to six months of Mirena use, the number of bleeding and spotting days may be increased and bleeding patterns may be irregular. Thereafter the number of bleeding and spotting days usually decreases but bleeding may remain irregular. If bleeding irregularities develop during prolonged treatment, appropriate diagnostic measures should be taken to rule out endometrial pathology.
Amenorrhea develops in approximately 20% of Mirena users by one year. The possibility of pregnancy should be considered if menstruation does not occur within six weeks of the onset of previous menstruation. Once pregnancy has been excluded, repeated pregnancy tests are generally not necessary in amenorrheic women unless indicated, for example, by other signs of pregnancy or by pelvic pain [see Clinical Studies (14.1)].
In most women with heavy menstrual bleeding, the number of bleeding and spotting days may also increase during the initial months of therapy but usually decrease with continued use; the volume of blood loss per cycle progressively becomes reduced [see Clinical Studies (14.2)].
5.6 Embedment
Embedment of Mirena in the myometrium may occur. Embedment may decrease contraceptive effectiveness and result in pregnancy [see Warnings and Precautions (5.1 and5.2)]. An embedded Mirena should be removed. Embedment can result in difficult removal and, in some cases surgical removal may be necessary.
5.7 Perforation
Perforation or penetration of the uterine wall or cervix may occur during insertion although the perforation may not be detected until some time later. If perforation occurs, pregnancy may result [see Warnings and Precautions (5.1 and5.2)]. Mirena must be located and removed; surgery may be required. Delayed detection of perforation may result in migration outside the uterine cavity, adhesions, peritonitis, intestinal perforations, intestinal obstruction, abscesses and erosion of adjacent viscera.
Clinical trials with Mirena excluded breast-feeding women. An interim analysis from a large postmarketing safety study shows an increased risk of perforation in lactating women. The risk of perforation may be increased in women with fixed retroverted uteri, and during the postpartum period. To decrease the risk of perforation postpartum, Mirena insertion should be delayed a minimum of 6 weeks after delivery or until uterine involution is complete. If involution is substantially delayed, consider waiting until 12 weeks postpartum. Inserting Mirena immediately after first trimester abortion is not known to increase the risk of perforation, but insertion after second trimester abortion should be delayed until uterine involution is complete.
5.8 Expulsion
Partial or complete expulsion of Mirena may occur [see Warnings and Precautions (5.13)].
Symptoms of the partial or complete expulsion of any lUD may include bleeding or pain. However, the system can be expelled from the uterine cavity without the woman noticing it, resulting in the loss of contraceptive protection. Partial expulsion may decrease the effectiveness of Mirena. As menstrual flow typically decreases after the first 3 to 6 months of Mirena use, an increase of menstrual flow may be indicative of an expulsion. If expulsion has occurred, Mirena may be replaced within 7 days of a menstrual period after pregnancy has been ruled out.
5.9 Ovarian Cysts
Since the contraceptive effect of Mirena is mainly due to its local effect, ovulatory cycles with follicular rupture usually occur in women of fertile age using Mirena. Sometimes atresia of the follicle is delayed and the follicle may continue to grow. Enlarged follicles have been diagnosed in about 12% of the subjects using Mirena. Most of these follicles are asymptomatic, although some may be accompanied by pelvic pain or dyspareunia. In most cases the enlarged follicles disappear spontaneously during two to three months observation. Persistent enlarged follicles should be evaluated. Surgical intervention is not usually required.
5.10 Breast Cancer
Women who currently have or have had breast cancer, or have a suspicion of breast cancer, should not use hormonal contraception because breast cancer is a hormone-sensitive tumor.
Spontaneous reports of breast cancer have been received during postmarketing experience with Mirena. Because spontaneous reports are voluntary and from a population of uncertain size, it is not possible to use postmarketing data to reliably estimate the frequency or establish causal relationship to drug exposure. Two observational studies have not provided evidence of an increased risk of breast cancer during the use of Mirena.
5.11 Patient Evaluation and Clinical Considerations
- •
- A complete medical and social history, including that of the partner, should be obtained to determine conditions that might influence the selection of an IUD for contraception [see Contraindications (4)].
- •
- Special attention must be given to ascertaining whether the woman is at increased risk of infection (for example, leukemia, acquired immune deficiency syndrome (AIDS), I.V. drug abuse), or has a history of PID unless there has been a subsequent intrauterine pregnancy. Mirena is contraindicated in these women.
- •
- A physical examination should include a pelvic examination, a Pap smear, examination of the breasts, and appropriate tests for any other forms of genital or other sexually transmitted diseases, such as gonorrhea and chlamydia laboratory evaluations, if indicated. Use of Mirena in patients with vaginitis or cervicitis should be postponed until proper treatment has eradicated the infection and until it has been shown that the cervicitis is not due to gonorrhea or chlamydia [see Contraindications (4)].
- •
- Irregular bleeding may mask symptoms and signs of endometrial polyps or cancer. Because irregular bleeding/spotting is common during the first months of Mirena use, exclude endometrial pathology prior to the insertion of Mirena in women with persistent or uncharacteristic bleeding. If unexplained bleeding irregularities develop during the prolonged use of Mirena, appropriate diagnostic measures should be taken [see Warnings and Precautions (5.5)].
- •
- The healthcare provider should determine that the patient is not pregnant. The possibility of insertion of Mirena in the presence of an existing undetermined pregnancy is reduced if insertion is performed within 7 days of the onset of a menstrual period. Mirena can be replaced by a new system at any time in the cycle. Mirena can be inserted immediately after first trimester abortion.
- •
- Mirena should not be inserted until 6 weeks postpartum or until involution of the uterus is complete in order to reduce the incidence of perforation and expulsion. If involution is substantially delayed, consider waiting until 12 weeks postpartum [see Warnings and Precautions (5.7)].
- •
- Patients with certain types of valvular or congenital heart disease and surgically constructed systemic-pulmonary shunts are at increased risk of infective endocarditis. Use of Mirena in these patients may represent a potential source of septic emboli. Patients with known congenital heart disease who may be at increased risk should be treated with appropriate antibiotics at the time of insertion and removal.
- •
- Patients requiring chronic corticosteroid therapy or insulin for diabetes should be monitored with special care for infection.
Mirena should be used with caution in patients who have:
- •
- Coagulopathy or are receiving anticoagulants
- •
- Migraine, focal migraine with asymmetrical visual loss or other symptoms indicating transient cerebral ischemia
- •
- Exceptionally severe headache
- •
- Marked increase of blood pressure
- •
- Severe arterial disease such as stroke or myocardial infarction.
5.12 Insertion Precautions
- •
- Observe strict asepsis during insertion. The presence of organisms capable of establishing PID cannot be determined by appearance, and IUD insertion may be associated with introduction of vaginal bacteria into the uterus. Administration of antibiotics may be considered, but the utility of this treatment is unknown.
- •
- Carefully sound the uterus prior to Mirena insertion to determine the degree of patency of the endocervical canal and the internal os, and the direction and depth of the uterine cavity. In occasional cases, severe cervical stenosis may be encountered. Do not use excessive force to overcome this resistance.
- •
- Fundal positioning of Mirena is important to prevent expulsion and maximize efficacy. Therefore, follow the instructions for the insertion carefully.
- •
- If the patient develops decreased pulse, perspiration, or pallor, have her remain supine until these signs resolve. Insertion may be associated with some pain and/or bleeding. Syncope, bradycardia, or other neurovascular episodes may occur during insertion of Mirena, especially in patients with a predisposition to these conditions or cervical stenosis.
5.13 Continuation and Removal
- •
- Reexamine and evaluate patients 4 to 12 weeks after insertion and once a year thereafter, or more frequently if clinically indicated.
- •
- If the threads are not visible, they may have retracted into the uterus or broken, or Mirena may have broken, perforated the uterus, or been expelled [see Warnings and Precautions (5.7 and 5.8)]. If the length of the threads has changed from the length at time of insertion, the system may have become displaced. Pregnancy must be excluded and the location of Mirena verified, for example, by sonography, X-ray, or by gentle exploration of the uterine cavity with a probe. If Mirena is displaced, remove it. A new Mirena may be inserted at that time or during the next menses if it is certain that conception has not occurred. If Mirena is in place with no evidence of perforation, no intervention is indicated.
- •
- Promptly examine users with complaints of pain, odorous discharge, unexplained bleeding [see Warnings and Precautions (5.5)], fever, genital lesions or sores.
- •
- Consider the possibility of ectopic pregnancy in the case of lower abdominal pain especially in association with missed periods or if an amenorrheic woman starts bleeding [see Warnings and Precautions (5.1)].
In the event a pregnancy is confirmed during Mirena use:
- •
- Determine whether pregnancy is ectopic and, if so, take appropriate measures.
- •
- Inform patient of the risks of leaving Mirena in place or removing it during pregnancy and of the lack of data on long-term effects on the offspring of women who have had Mirena in place during conception or gestation [see Warnings and Precautions (5.2)].
- •
- If possible, Mirena should be removed after the patient has been warned of the risks of removal. If removal is difficult, the patient should be counseled and offered pregnancy termination.
- •
- If Mirena is left in place, the patient's course should be followed closely.
In the event of a sexually transmitted disease during Mirena use:
Should the patient's relationship cease to be mutually monogamous, or should her partner become HIV positive, or acquire a sexually transmitted disease, she should be instructed to report this change to her clinician immediately. The use of a barrier method as a partial protection against acquiring sexually transmitted diseases should be strongly recommended. Removal of Mirena should be considered.
Mirena should be removed for the following medical reasons:
- •
- New onset menorrhagia and/or metrorrhagia producing anemia
- •
- Sexually transmitted disease
- •
- Pelvic infection; endometritis
- •
- Symptomatic genital actinomycosis
- •
- Intractable pelvic pain
- •
- Severe dyspareunia
- •
- Pregnancy
- •
- Endometrial or cervical malignancy
- •
- Uterine or cervical perforation.
Removal of the system should also be considered if any of the following conditions arise for the first time:
- •
- Migraine, focal migraine with asymmetrical visual loss or other symptoms indicating transient cerebral ischemia
- •
- Exceptionally severe headache
- •
- Jaundice
- •
- Marked increase of blood pressure
- •
- Severe arterial disease such as stroke or myocardial infarction.
Removal may be associated with some pain and/or bleeding or neurovascular episodes.
6 ADVERSE REACTIONS
The following most serious adverse reactions associated with the use of Mirena are discussed in greater detail in the Warnings and Precautions section (5):
- •
- Ectopic Pregnancy [see Warnings and Precautions (5.1)]
- •
- Intrauterine Pregnancy [see Warnings and Precautions (5.2)]
- •
- Group A streptococcal sepsis (GAS) [see Warnings and Precautions (5.3)]
- •
- Pelvic Inflammatory Disease [see Warnings and Precautions (5.4)]
- •
- Embedment [see Warnings and Precautions (5.6)]
- •
- Perforation [see Warnings and Precautions (5.7)]
- •
- Breast Cancer [see Warnings and Precautions (5.10)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data provided reflect the experience with the use of Mirena in the adequate and well-controlled studies for contraception (n=2,339) and heavy menstrual bleeding (n=80). For the contraception indication, Mirena was compared to a copper IUD (n=1,855), to another formulation of levonorgestrel intrauterine system (n=390) and to a combined oral contraceptive (n=94) in women 18 to 35 years old. The data cover more than 92,000 woman-months of exposure. For the treatment of heavy menstrual bleeding indication (n=80), the subjects included women aged 26 to 50 with confirmed heavy bleeding and exposed for a median of 183 treatment days of Mirena (range 7 to 295 days). The frequencies of reported adverse drug reactions represent crude incidences.
The adverse reactions seen across the 2 indications overlapped, and are reported using the frequencies from the contraception studies.
The most common adverse reactions (≥5% users) are uterine/vaginal bleeding alterations (51.9%), amenorrhea (23.9%), intermenstrual bleeding and spotting (23.4%), abdominal/pelvic pain (12.8%), ovarian cysts (12%), headache/migraine (7.7%), acne (7.2%), depressed/altered mood (6.4%), menorrhagia (6.3%), breast tenderness/pain (4.9%), vaginal discharge (4.9%) and IUD expulsion (4.9%).
Other relevant adverse reactions occurring in <5% of subjects include nausea, nervousness, vulvovaginitis, dysmenorrhea, back pain, weight increase, decreased libido, cervicitis/Papanicolaou smear normal/class II, hypertension, dyspareunia, anemia, alopecia, skin disorders including eczema, pruritus, rash and urticaria, abdominal distention, hirsutism and edema.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Mirena: device breakage and angioedema. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
Drugs or herbal products that induce enzymes, including CYP3A4, that metabolize progestins may decrease the serum concentrations of progestins.
Some drugs or herbal products that may decrease the serum concentration of levonorgestrel include:
- •
- barbiturates
- •
- bosentan
- •
- carbamazepine
- •
- felbamate
- •
- griseofulvin
- •
- oxcarbazepine
- •
- phenytoin
- •
- rifampin
- •
- St. John’s wort
- •
- topiramate.
Significant changes (increase or decrease) in the serum concentrations of the progestin have been noted in some cases of co-administration with HIV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
Consult the labeling of all concurrently used drugs to obtain further information about interactions with Mirena or the potential for enzyme alterations.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Many studies have found no harmful effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted with progestin-only pills have not demonstrated significant adverse effects. [Also see Contraindications (4), Warnings and Precautions (5.1 and 5.2).]
8.3 Nursing Mothers
In general, no adverse effects have been found on breastfeeding performance or on the health, growth, or development of the infant. However, isolated postmarketing cases of decreased milk production have been reported. Small amounts of progestins pass into the breast milk of nursing mothers, resulting in detectable steroid levels in infant serum. [Also,see Warnings and Precautions (5.7 ).]
8.4 Pediatric Use
Safety and efficacy of Mirena have been established in women of reproductive age. Use of this product before menarche is not indicated.
8.5 Geriatric Use
Mirena has not been studied in women over age 65 and is not currently approved for use in this population.
8.6 Hepatic Impairment
No studies were conducted to evaluate the effect of hepatic disease on the disposition of levonorgestrel released from Mirena [see Contraindications (4)].
11 DESCRIPTION
Mirena is intended to provide an initial release rate of 20 mcg/day of levonorgestrel
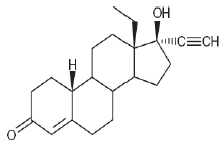
Levonorgestrel USP, (-)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one, the active ingredient in Mirena, has a molecular weight of 312.4, a molecular formula of C21H28O2, and the following structural formula:
11.1 Mirena
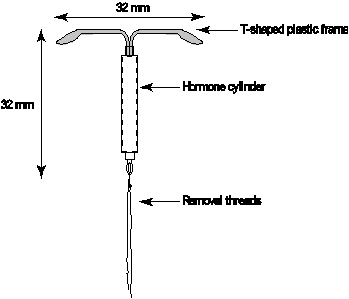
Mirena (levonorgestrel-releasing intrauterine system) consists of a T-shaped polyethylene frame (T-body) with a steroid reservoir (hormone elastomer core) around the vertical stem. The reservoir consists of a white or almost white cylinder, made of a mixture of levonorgestrel and silicone (polydimethylsiloxane), containing a total of 52 mg levonorgestrel. The reservoir is covered by a semi-opaque silicone (polydimethylsiloxane) membrane. The T-body is 32 mm in both the horizontal and vertical directions. The polyethylene of the T-body is compounded with barium sulfate, which makes it radiopaque. A monofilament brown polyethylene removal thread is attached to a loop at the end of the vertical stem of the T-body.
11.2 Inserter
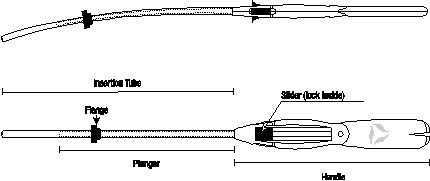
Mirena is packaged sterile within an inserter. The inserter, which is used for insertion of Mirena into the uterine cavity, consists of a symmetric two-sided body and slider that are integrated with flange, lock, pre-bent insertion tube and plunger. Once Mirena is in place, the inserter is discarded.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
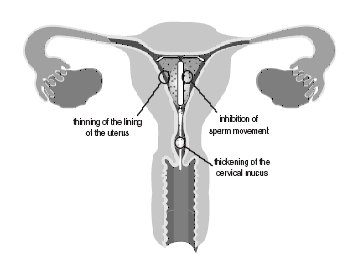
The local mechanism by which continuously released levonorgestrel enhances contraceptive effectiveness of Mirena has not been conclusively demonstrated. Studies of Mirena prototypes have suggested several mechanisms that prevent pregnancy: thickening of cervical mucus preventing passage of sperm into the uterus, inhibition of sperm capacitation or survival, and alteration of the endometrium.
12.2 Pharmacodynamics
Mirena has mainly local progestogenic effects in the uterine cavity. The high local levels of levonorgestrel1 lead to morphological changes including stromal pseudodecidualization, glandular atrophy, a leukocytic infiltration and a decrease in glandular and stromal mitoses.
Ovulation is inhibited in some women using Mirena. In a 1-year study approximately 45% of menstrual cycles were ovulatory and in another study after 4 years 75% of cycles were ovulatory.
12.3 Pharmacokinetics
Absorption
Low doses of levonorgestrel are administered into the uterine cavity with the Mirena intrauterine delivery system. Initially, levonorgestrel is released at a rate of approximately 20 mcg/day. This rate decreases progressively to half that value after 5 years. A stable serum concentration, without peaks and troughs, of levonorgestrel of 150–200 pg/mL occurs after the first few weeks following insertion of Mirena. Levonorgestrel concentrations after long-term use of 12, 24, and 60 months were 180±66 pg/mL, 192±140 pg/mL, and 159±59 pg/mL, respectively.
Distribution
The apparent volume of distribution of levonorgestrel is reported to be approximately 1.8 L/kg. It is about 97.5 to 99% protein-bound, principally to sex hormone binding globulin (SHBG) and, to a lesser extent, serum albumin.
Metabolism
Following absorption, levonorgestrel is conjugated at the 17β-OH position to form sulfate conjugates and, to a lesser extent, glucuronide conjugates in serum. Significant amounts of conjugated and unconjugated 3α, 5β- tetrahydrolevonorgestrel are also present in serum, along with much smaller amounts of 3α, 5α-tetrahydrolevonorgestrel and 16βhydroxylevonorgestrel. Levonorgestrel and its phase I metabolites are excreted primarily as glucuronide conjugates. Metabolic clearance rates may differ among individuals by several-fold, and this may account in part for wide individual variations in levonorgestrel concentrations seen in individuals using levonorgestrel–containing contraceptive products.
Excretion
About 45% of levonorgestrel and its metabolites are excreted in the urine and about 32% are excreted in feces, mostly as glucuronide conjugates. The elimination half-life of levonorgestrel after daily oral doses is approximately 17 hours.
Specific Populations
Pediatric: Safety and efficacy of Mirena have been established in women of reproductive age. Use of this product before menarche is not indicated.
Geriatric: Mirena has not been studied in women over age 65 and is not currently approved for use in this population.
Race: No studies have evaluated the effect of race on pharmacokinetics of Mirena.
Hepatic Impairment: No studies were conducted to evaluate the effect of hepatic disease on the disposition of Mirena.
Renal Impairment: No formal studies were conducted to evaluate the effect of renal disease on the disposition of Mirena.
Drug-Drug Interactions
No drug-drug interaction studies were conducted with Mirena [see Drug Interactions (7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Long-term studies in animals to assess the carcinogenic potential of levonorgestrel releasing intrauterine system have not been performed. There is no evidence of increased risk of cancer with short-term use of progestins. There was no increase in tumorigenicity following parenteral administration of levonorgestrel to rats for 2 years at approximately 5 mcg/day, or following oral administration to dogs for 7 years at up to 0.125 mg/kg/day, or to rhesus monkeys for 10 years at up to 250 mcg/kg/day. In another 7 year dog study, oral administration of levonorgestrel at 0.5 mg/kg/day did increase the number of mammary adenomas in treated dogs compared to controls. There were no malignancies. The nonclinical doses above are respectively 16, 200, 240 and 810 times the release rate of levonorgestrel by Mirena (20 mcg/day), based on body surface area [see Warnings and Precautions (5.10)].
13.2 Animal Toxicology and/or Pharmacology
Carcinogenicity
Long-term studies in animals to assess the carcinogenic potential of levonorgestrel releasing intrauterine system have not been performed. There is no evidence of increased risk of cancer with short-term use of progestins. There was no increase in tumorigenicity following parenteral administration of levonorgestrel to rats for 2 years at approximately 5 mcg/day, or following oral administration to dogs for 7 years at up to 0.125 mg/kg/day, or to rhesus monkeys for 10 years at up to 250 mcg/kg/day. In another 7 year dog study, oral administration of levonorgestrel at 0.5 mg/kg/day did increase the number of mammary adenomas in treated dogs compared to controls. There were no malignancies. The nonclinical doses above are respectively 16, 200, 240 and 810 times the release rate of levonorgestrel by Mirena (20 mcg/day), based on body surface area [see Warnings and Precautions (5.10)].
14 CLINICAL STUDIES
14.1 Clinical Trials on Intrauterine Contraception
Mirena has been studied for safety and efficacy in two large clinical trials in Finland and Sweden. In study sites having verifiable data and informed consent, 1,169 women 18 to 35 years of age at enrollment used Mirena for up to 5 years, for a total of 45,000 women-months of exposure. Subjects had previously been pregnant, had no history of ectopic pregnancy, had no history of pelvic inflammatory disease over the preceding 12 months, were predominantly Caucasian, and over 70% of the participants had previously used IUDs (intrauterine devices). The reported 12-month pregnancy rates were less than or equal to 0.2 per 100 women (0.2%) and the cumulative 5-year pregnancy rate was approximately 0.7 per 100 women (0.7%).
About 80% of women wishing to become pregnant conceived within 12 months after removal of Mirena.
14.2 Clinical Trial on Heavy Menstrual Bleeding
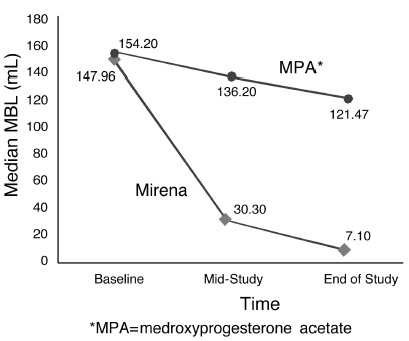
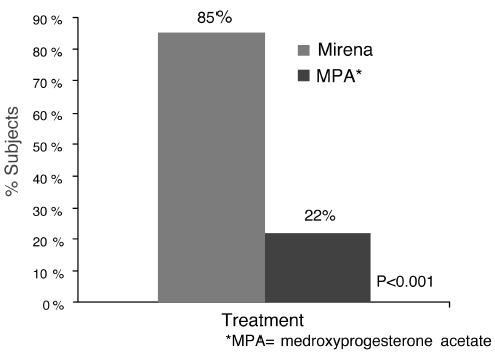
The efficacy of Mirena in the treatment of heavy menstrual bleeding was studied in a randomized, open-label, active-control, parallel-group trial comparing Mirena (n=79) to an approved therapy, medroxyprogesterone acetate (MPA) (n=81), over 6 cycles. The subjects included reproductive-aged women in good health, with no contraindications to the drug products and with confirmed heavy menstrual bleeding (≥ 80 mL menstrual blood loss [MBL]) determined using the alkaline hematin method. Excluded were women with organic or systemic conditions that may cause heavy uterine bleeding (except small fibroids, with total volume not > 5 mL). Treatment with Mirena showed a statistically significantly greater reduction in MBL (see Figure 10) and a statistically significantly greater number of subjects with successful treatment (see Figure 11). Successful treatment was defined as proportion of subjects with (1) end-of-study MBL < 80 mL and (2) a ≥ 50% decrease in MBL from baseline to end-of-study.
15 REFERENCES
1Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue Concentrations of Levonorgestrel in Women Using a Levonorgestrel-releasing IUD. Clinical Endocrinol 1982;17:529-536.
16 HOW SUPPLIED/STORAGE AND HANDLING
Mirena (levonorgestrel-releasing intrauterine system), containing a total of 52 mg levonorgestrel, is available in a carton of one sterile unit NDC# 50419-421-01. Each Mirena is packaged together with an inserter in a thermoformed blister package with a peelable lid.
Mirena is supplied sterile. Mirena is sterilized with ethylene oxide. Do not resterilize. For single use only. Do not use if the inner package is damaged or open. Insert before the end of the month shown on the label.
Store at 25°C (77°F); with excursions permitted between 15–30°C (59–86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
- •
- Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases (STDs).
- •
- Prior to insertion, give the patient the Patient Information Booklet. She should be given the opportunity to read the information and discuss fully any questions she may have concerning Mirena as well as other methods of contraception and therapies for heavy menstrual bleeding. Also, advise the patient that the prescribing information is available to her upon request.
- •
- Inform the patient that irregular or prolonged bleeding and spotting, and/or cramps may occur during the first few weeks after insertion. If her symptoms continue or are severe she should report them to her healthcare provider.
- •
- Instruct the patient to contact her healthcare provider if she experiences any of the following:
- •
- A stroke or heart attack
- •
- Develops very severe or migraine headaches
- •
- Unexplained fever
- •
- Yellowing of the skin or whites of the eyes, as these may be signs of serious liver problems
- •
- She thinks she is pregnant
- •
- Pelvic pain or pain during sex
- •
- She or her partner becomes HIV positive
- •
- She might be exposed to sexually transmitted diseases (STDs)
- •
- Unusual vaginal discharge or genital sores
- •
- Severe vaginal bleeding or bleeding that lasts a long time, or if she misses a menstrual period
- •
- Cannot feel Mirena's threads.
Instruct the patient on how to check after her menstrual period to make certain that the threads still protrude from the cervix and caution her not to pull on the threads and displace Mirena. Inform her that there is no contraceptive protection if Mirena is displaced or expelled.
FDA-Approved Patient Information
PATIENT INFORMATION
Mirena® (Mur-ā-nah)
(levonorgestrel-releasing intrauterine system)
Mirena does not protect against HIV infection (AIDS) and other sexually transmitted diseases (STDs).
Read this Patient Information carefully before you decide if Mirena is right for you. This information does not take the place of talking with your gynecologist or other healthcare provider who specializes in women’s health. If you have any questions about Mirena, ask your healthcare provider. You should also learn about other birth control methods to choose the one that is best for you.
What is Mirena?
- •
- Mirena is a hormone-releasing system placed in your uterus to prevent pregnancy for up to 5 years.
- •
- Mirena can also lessen menstrual blood loss in women who have heavy menstrual flow and who also want to use a birth control method that is placed in the uterus to prevent pregnancy.
- •
- Mirena is recommended for women who have had at least one child.
Mirena is T-shaped. It is made of flexible plastic and contains a progestin hormone called levonorgestrel that is often used in birth control pills. Mirena does not contain estrogen. Mirena releases the hormone into the uterus. Only small amounts of the hormone enter your blood.
Two threads are attached to the stem of Mirena. The threads are the only part of Mirena you can feel when Mirena is in your uterus.
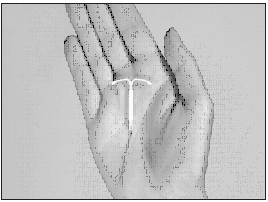
Mirena is small...
and flexible
What if I need birth control for more than 5 years?
Mirena must be removed after 5 years. Your healthcare provider can insert a new Mirena during the same office visit if you choose to continue using Mirena.
What if I change my mind about birth control and want to become pregnant in less than 5 years?
Your healthcare provider can remove Mirena at any time. You may become pregnant as soon as Mirena is removed. About 8 out of 10 women who want to become pregnant will become pregnant some time in the first year after Mirena is removed.
How does Mirena work?
It is not known exactly how Mirena works. Mirena may work in several ways. It may thicken your cervical mucus, thin the lining of your uterus, inhibit sperm movement and reduce sperm survival. Mirena may stop release of your egg from your ovary, but this is not the way it works in most cases. Most likely, these actions work together to prevent pregnancy. Mirena can cause your menstrual bleeding to be less by thinning the lining of the uterus.
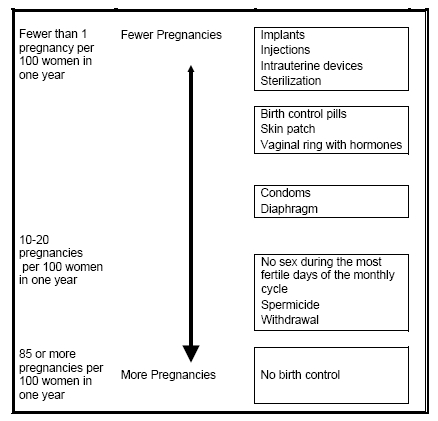
How well does Mirena work for contraception?
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are in the box at the top of the chart. Mirena, an intrauterine device, is in the box at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
How well does Mirena work for heavy menstrual bleeding?
In the clinical trial performed in women with heavy menstrual bleeding and treated with Mirena, almost 9 out of 10 were treated successfully and their blood loss was reduced by more than half.
Who might use Mirena?
You might choose Mirena if you:
- •
- Want birth control that provides a low chance of getting pregnant (less than 1 in 100)
- •
- Want birth control that is reversible
- •
- Want a birth control method that does not require taking it daily
- •
- Have had at least one child
- •
- Want treatment for heavy periods and want to use a birth control method that is placed in the uterus to prevent pregnancy.
Who should not use Mirena?
Do not use Mirena if you:
- •
- Might be pregnant
- •
- Have had a serious pelvic infection called pelvic inflammatory disease (PID) unless you have had a normal pregnancy after the infection went away
- •
- Have an untreated pelvic infection now
- •
- Have had a serious pelvic infection in the past 3 months after a pregnancy
- •
- Can get infections easily. For example, if you have:
- •
- More than one sexual partner or your partner has more than one partner
- •
- Problems with your immune system
- •
- Intravenous drug abuse.
- •
- Have or suspect you might have cancer of the uterus or cervix
- •
- Have bleeding from the vagina that has not been explained
- •
- Have liver disease or liver tumor
- •
- Have breast cancer now or in the past or suspect you have breast cancer
- •
- Have an intrauterine device in your uterus already
- •
- Have a condition of the uterus that changes the shape of the uterine cavity, such as large fibroid tumors
- •
- Are allergic to levonorgestrel, silicone, or polyethylene.
Before having Mirena placed, tell your healthcare provider if you:
- •
- Have had a heart attack
- •
- Have had a stroke
- •
- Were born with heart disease or have problems with your heart valves
- •
- Have problems with blood clotting or take medicine to reduce clotting
- •
- Have high blood pressure
- •
- Recently had a baby or if you are breast feeding
- •
- Have diabetes (high blood sugar)
- •
- Use corticosteroid medications on a long-term basis
- •
- Have severe migraine headaches.
How is Mirena placed?
First, your healthcare provider will examine your pelvis to find the exact position of your uterus. Your healthcare provider will then clean your vagina and cervix with an antiseptic solution, and slide a thin plastic tube containing Mirena into your uterus. Your healthcare provider will then remove the plastic tube, and leave Mirena in your uterus. Your healthcare provider will cut the threads to the right length. Placement takes only a few minutes during an office visit.
You may experience pain, bleeding or dizziness during and after placement. If these symptoms do not pass 30 minutes after placement, Mirena may not have been placed correctly. Your healthcare provider will examine you to see if Mirena needs to be removed or replaced.
Should I check that Mirena is in the proper position?
Yes, you should check that Mirena is in proper position by feeling the removal threads. You should do this after each menstrual period. First, wash your hands with soap and water. Feel for the threads at the top of your vagina with your clean fingers. The threads are the only part of Mirena you should feel when Mirena is in your uterus. Be careful not to pull on the threads. If you feel more than just the threads, Mirena is not in the right position and may not prevent pregnancy. Call your healthcare provider to have it removed. If you cannot feel the threads at all, ask your healthcare provider to check that Mirena is still in the right place. In either case, use a non-hormonal birth control method (such as condoms or spermicide) until otherwise advised by your healthcare provider.
How soon after placement of Mirena should I return to my healthcare provider?
Call your healthcare provider if you have any questions or concerns (see "When should I call my healthcare provider"). Otherwise, you should return to your healthcare provider for a follow-up visit 4 to 12 weeks after Mirena is placed to make sure that Mirena is in the right position.
Can I use tampons with Mirena?
Tampons may be used with Mirena.
What if I become pregnant while using Mirena?
Call your healthcare provider right away if you think you are pregnant. If you get pregnant while using Mirena, you may have an ectopic pregnancy. This means that the pregnancy is not in the uterus. Unusual vaginal bleeding or abdominal pain may be a sign of ectopic pregnancy.
Ectopic pregnancy is a medical emergency that often requires surgery. Ectopic pregnancy can cause internal bleeding, infertility, and even death.
There are also risks if you get pregnant while using Mirena and the pregnancy is in the uterus. Severe infection, miscarriage, premature delivery, and even death can occur with pregnancies that continue with an intrauterine device (IUD). Because of this, your healthcare provider may try to remove Mirena, even though removing it may cause a miscarriage. If Mirena cannot be removed, talk with your healthcare provider about the benefits and risks of continuing the pregnancy.
If you continue your pregnancy, see your healthcare provider regularly. Call your healthcare provider right away if you get flu-like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge, or fluid leaking from your vagina. These may be signs of infection.
It is not known if Mirena can cause long-term effects on the fetus if it stays in place during a pregnancy.
How will Mirena change my periods?
For the first 3 to 6 months, your monthly period may become irregular and the number of bleeding days may increase at first. You may also have frequent spotting or light bleeding. A few women have heavy bleeding during this time. After your body adjusts, the number of bleeding days is likely to lessen, and you may even find that your periods stop altogether.
In some women with heavy bleeding, the total blood loss per cycle progressively decreases with continued use. The number of spotting and bleeding days may initially increase but then typically decreases in the months that follow.
Is it safe to breast-feed while using Mirena?
You may use Mirena when you are breastfeeding if more than six weeks have passed since you had your baby. If you are breastfeeding, Mirena is not likely to affect the quality or amount of your breast milk or the health of your nursing baby. However, isolated cases of decreased milk production have been reported among women using progestin-only birth control pills.
Will Mirena interfere with sexual intercourse?
You and your partner should not feel Mirena during intercourse. Mirena is placed in the uterus, not in the vagina. Sometimes male partners feel the threads.
What are the possible side effects of using Mirena?
Mirena can cause serious side effects including:
- •
- Pelvic inflammatory disease (PID). Some IUD users get a serious pelvic infection called pelvic inflammatory disease. PID is usually sexually transmitted. You have a higher chance of getting PID if you or your partner have sex with other partners. PID can cause serious problems such as infertility, ectopic pregnancy or pelvic pain that does not go away. PID is usually treated with antibiotics. More serious cases of PID may require surgery. A hysterectomy (removal of the uterus) is sometimes needed. In rare cases, infections that start as PID can even cause death.
- •
- Tell your healthcare provider right away if you have any of these signs of PID: long-lasting or heavy bleeding, unusual vaginal discharge, low abdominal (stomach area) pain, painful sex, chills, or fever.
- •
- Life-threatening infection. Life-threatening infection can occur within the first few days after Mirena is placed. Call your healthcare provider if you develop severe pain within a few hours after Mirena is placed.
- •
- Embedment. Mirena may become attached to the uterine wall. This is called embedment. If embedment happens, Mirena may no longer prevent pregnancy and you may need surgery to have it removed.
- •
- Perforation. Mirena may go through the uterus. This is called perforation. If your uterus is perforated, Mirena may no longer prevent pregnancy. It may move outside the uterus and can cause internal scarring, infection, or damage to other organs, and you may need surgery to have Mirena removed. The risk of perforation is increased in breastfeeding women.
Common side effects of Mirena include:
- •
- Pain, bleeding or dizziness during and after placement. If these symptoms do not stop 30 minutes after placement, Mirena may not have been placed correctly. Your healthcare provider will examine you to see if Mirena needs to be removed or replaced.
- •
- Expulsion. Mirena may come out by itself. This is called expulsion. You may become pregnant if Mirena comes out. If you notice that Mirena has come out, use a backup birth control method like condoms and call your healthcare provider.
- •
- Missed menstrual periods. About 2 out of 10 women stop having periods after 1 year of Mirena use. If you do not have a period for 6 weeks during Mirena use, call your healthcare provider. When Mirena is removed, your menstrual periods will come back.
- •
- Changes in bleeding. You may have bleeding and spotting between menstrual periods, especially during the first 3 to 6 months. Sometimes the bleeding is heavier than usual at first. However, the bleeding usually becomes lighter than usual and may be irregular. Call your healthcare provider if the bleeding remains heavier than usual or if the bleeding becomes heavy after it has been light for a while.
- •
- Cyst on the ovary. About 12 out of 100 women using Mirena develop a cyst on the ovary. These cysts usually disappear on their own in a month or two. However, cysts can cause pain and sometimes cysts will need surgery.
This is not a complete list of possible side effects with Mirena. For more information, ask your healthcare provider.
Call your doctor for medical advice about side effects. You may report side effects to the manufacturer at 1-888-842-2937, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
After Mirena has been placed, when should I call my healthcare provider?
Call your healthcare provider if you have any concerns about Mirena. Be sure to call if you:
- •
- Think you are pregnant.
- •
- Have pelvic pain or pain during sex.
- •
- Have unusual vaginal discharge or genital sores.
- •
- Have unexplained fever.
- •
- Might be exposed to sexually transmitted diseases (STDs).
- •
- Cannot feel Mirena 's threads.
- •
- Develop very severe or migraine headaches.
- •
- Have yellowing of the skin or whites of the eyes. These may be signs of liver problems.
- •
- Have a stroke or heart attack.
- •
- Or your partner becomes HIV positive.
- •
- Have severe vaginal bleeding or bleeding that lasts a long time.
General advice about prescription medicines
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. This leaflet summarizes the most important information about Mirena. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider for information about Mirena that is written for health providers.
© 2013, Bayer HealthCare Pharmaceuticals Inc. All rights reserved.
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
This patient information booklet was updated February 2013.
Mirena (levonorgestrel-releasing intrauterine system) Carton
NDC 50419-421-01
1 Sterile Unit
IMPORTANT: To be inserted in the uterus by
or under the supervision of a licensed clinician.
See physician insert for detailed instructions
for use.
Mirena
(levonorgestrel-releasing intrauterine system)
Rx only
— 52 mg levonorgestrel
— 1 sterile unit
— intrauterine use
| MIRENA
levonorgestrel intrauterine device |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bayer Schering Pharma Oy | 369758383 | MANUFACTURE(50419-421) | |