TIMENTIN- ticarcillin disodium and clavulanate potassium injection, powder, for solution
TIMENTIN- ticarcillin disodium and clavulanate potassium injection, solution
GlaxoSmithKline LLC
Reference Label Set Id: 2402ba3b-e06e-4974-0b99-cfff5cb2cb21
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TIMENTIN safely and effectively. See full prescribing information for TIMENTIN.
TIMENTIN (ticarcillin disodium and clavulanate potassium) for Injection TIMENTIN (ticarcillin disodium and clavulanate potassium) for Injection: Pharmacy Bulk Package TIMENTIN (ticarcillin disodium and clavulanate potassium) Injection: GALAXY Container Initial U.S. Approval: 1985 INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of TIMENTIN and other antibacterial drugs, TIMENTIN should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. TIMENTIN is a combination of a β‑lactam antibacterial and a β‑lactamase inhibitor indicated for the treatment of the following infections due to designated susceptible bacteria: DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSHistory of a serious hypersensitivity reaction (anaphylaxis or Stevens-Johnson syndrome) to TIMENTIN or to other β‑lactams (e.g., penicillins and cephalosporins). (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (≥1%) are rash, nausea, diarrhea, and phlebitis at injection site. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 6/2014 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
TIMENTIN® is indicated in the treatment of infections caused by susceptible isolates of the designated bacteria in the conditions listed below:
1.1 Septicemia
Septicemia (including bacteremia) caused by β‑lactamase–producing isolates of Klebsiella spp.*, Escherichia coli*, Staphylococcus aureus*, or Pseudomonas aeruginosa* (or other Pseudomonas species*)
1.2 Lower Respiratory Infections
Lower respiratory infections caused by β‑lactamase–producing isolates of S. aureus, Haemophilus influenzae*, or Klebsiella spp.*
1.3 Bone and Joint Infections
Bone and joint infections caused by β‑lactamase–producing isolates of S. aureus
1.4 Skin and Skin Structure Infections
Skin and skin structure infections caused by β‑lactamase–producing isolates of S. aureus, Klebsiella spp.*, or E. coli*
1.5 Urinary Tract Infections
Urinary tract infections (complicated and uncomplicated) caused by β‑lactamase–producing isolates of E. coli, Klebsiella spp., P. aeruginosa* (or other Pseudomonas spp.*), Citrobacter spp.*, Enterobacter cloacae*, Serratia marcescens*, or S. aureus*
1.6 Gynecologic Infections
Endometritis caused by β‑lactamase–producing isolates of Prevotella melaninogenicus*, Enterobacter spp. (including E. cloacae*), E. coli, Klebsiella pneumoniae*, S. aureus, or Staphylococcus epidermidis
1.7 Intra-abdominal Infections
Peritonitis caused by β‑lactamase–producing isolates of E. coli, K. pneumoniae, or Bacteroides fragilis* group
* Efficacy for this organism in this organ system was studied in fewer than 10 infections.
1.8 Usage
To reduce the development of drug‑resistant bacteria and maintain the effectiveness of TIMENTIN and other antibacterial drugs, TIMENTIN should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Adults
The usual recommended dosage for systemic and urinary tract infections for adults is 3.1 grams of TIMENTIN (3 grams ticarcillin and 100 mg clavulanic acid) given every 4 to 6 hours.
For gynecologic infections, TIMENTIN should be administered as follows (based on ticarcillin content): Moderate infections, 200 mg/kg/day in divided doses every 6 hours; severe infections, 300 mg/kg/day in divided doses every 4 hours.
For patients weighing less than 60 kg, the recommended dosage is 200 to 300 mg/kg/day given in divided doses every 4 to 6 hours.
The duration of therapy depends upon the severity of infection. The usual duration is 10 to 14 days; however, in difficult and complicated infections, more prolonged therapy may be required.
2.2 Pediatric Patients (≥3 Months of Age)
Patients <60 kg: Mild to moderate infections, 200 mg/kg/day based on ticarcillin content in divided doses every 6 hours; severe infections, 300 mg/kg/day in divided doses every 4 hours.
Patients ≥60 kg: Mild to moderate infections, 3.1 grams every 6 hours; severe infections, 3.1 grams every 4 hours.
2.3 Renal Impairment
For patients with renal insufficiency, an initial loading dose of 3.1 grams should be followed by doses based on creatinine clearance and type of dialysis as indicated in Table 1.
|
Creatinine Clearance (mL/minute)a |
Dosageb |
|
Over 60 |
3 grams every 4 hours |
|
30 to 60 |
2 grams every 4 hours |
|
10 to 30 |
2 grams every 8 hours |
|
Less than 10 |
2 grams every 12 hours |
|
Less than 10 with hepatic dysfunction |
2 grams every 24 hours |
|
Patients on peritoneal dialysis |
3 grams every 12 hours |
|
Patients on hemodialysis |
2 grams every 12 hours supplemented with 3 grams after each dialysis |
a To calculate creatinine clearance1 from a serum creatinine value use the following formula:
Ccr = (140–Age) (weight in kg)/72 x Scr (mg/100 mL)
This is the calculated creatinine clearance for adult males; for females it is 15% less.
b Based on ticarcillin content.
2.4 Administration and Directions for Use
TIMENTIN should be administered by intravenous infusion over a 30-minute period.
Directions for Reconstitution and Further Dilution: 3.1‑gram Glass Vials: The 3.1‑gram vial should be reconstituted by adding approximately 13 mL of Sterile Water for Injection, USP, or Sodium Chloride Injection, USP, and shaking well. When dissolved, the concentration of ticarcillin will be approximately 200 mg/mL with a corresponding concentration of 6.7 mg/mL for clavulanic acid. The color of reconstituted solutions of TIMENTIN normally ranges from light to dark yellow, depending on concentration, duration, and temperature of storage.
The dissolved drug should be further diluted to desired volume using the recommended solution listed under Stability [see Dosage and Administration (2.5)] to a concentration between 10 mg/mL to 100 mg/mL.
Pharmacy Bulk Package: The container closure may be penetrated only one time utilizing a suitable sterile transfer device or dispensing set that allows measured distribution of the contents. A sterile substance that must be reconstituted prior to use may require a separate closure entry.
Restrict use of Pharmacy Bulk Packages to an aseptic area such as a laminar flow hood.
Reconstituted contents of the vial should be withdrawn immediately. However, if this is not possible, aliquoting operations must be completed within 4 hours of reconstitution. Discard the reconstituted stock solution 4 hours after initial entry.
Add 76 mL of Sterile Water for Injection, USP, or Sodium Chloride Injection, USP, to the 31‑gram Pharmacy Bulk Package and shake well. For ease of reconstitution, the diluent may be added in 2 portions. Each 1 mL of the resulting concentrated stock solution contains approximately 300 mg of ticarcillin and 10 mg of clavulanic acid.
The desired dosage should be withdrawn from the stock solution and further diluted to desired volume using the recommended solution listed under Stability [see Dosage and Administration (2.5)] to a concentration between 10 mg/mL to 100 mg/mL.
Directions for Intravenous Infusion: After reconstitution and further dilution and prior to administration, TIMENTIN should be inspected visually for particulate matter. If particulate matter is present, the solution should be discarded.
The solution of reconstituted drug may be administered over a 30-minute period by direct infusion or through a Y‑type intravenous infusion set. If this method of administration is used, it is advisable to temporarily discontinue the administration of any other solutions during the infusion of TIMENTIN.
When TIMENTIN is given in combination with another antimicrobial, such as an aminoglycoside, each drug should be given separately in accordance with the recommended dosage and routes of administration for each drug [see Drug Interactions (7.1)].
GALAXY® Container (PL 2040 Plastic): Prior to administration, TIMENTIN should be inspected visually for particulate matter. If particulate matter is present, the solution should be discarded.
Caution: Do not use plastic containers in series connections. Such use could result in an embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
Preparation for Administration: See How Supplied/Storage and Handling (16) for thawing and handling instructions:
- •
- Suspend the container from eyelet support.
- •
- Remove protector from outlet port at bottom of container.
- •
- Attach administration set. Refer to complete directions accompanying set.
2.5 Stability
NOTE: TIMENTIN is incompatible with Sodium Bicarbonate.
3.1‑gram Glass Vials: The concentrated stock solution at 200 mg/mL is stable for up to 6 hours at room temperature 21° to 24°C (70° to 75°F) or up to 72 hours under refrigeration 4°C (40°F).
If the concentrated stock solution (200 mg/mL) is held for up to 6 hours at room temperature 21° to 24°C (70° to 75°F) or up to 72 hours under refrigeration 4°C (40°F) and further diluted to a concentration between 10 mg/mL and 100 mg/mL with any of the diluents listed below, then the following stability periods apply.
|
STABILITY PERIOD |
||
|
(3.1‑gram Vials) |
||
|
Intravenous Solution (ticarcillin concentrations of 10 mg/mL to 100 mg/mL) |
Room Temperature 21° to 24°C (70° to 75°F) |
Refrigerated 4°C (40°F) |
|
Dextrose Injection 5%, USP |
24 hours |
3 days |
|
Sodium Chloride Injection, USP |
24 hours |
7 days |
|
Lactated Ringer’s Injection, USP |
24 hours |
7 days |
If the concentrated stock solution (200 mg/mL) is stored for up to 6 hours at room temperature and then further diluted to a concentration between 10 mg/mL and 100 mg/mL, solutions of Sodium Chloride Injection, USP, and Lactated Ringer’s Injection, USP, may be stored frozen –18°C (0°F) for up to 30 days. Solutions prepared with Dextrose Injection 5%, USP, may be stored frozen –18°C (0°F) for up to 7 days. All thawed solutions should be used within 8 hours or discarded. Once thawed, solutions should not be refrozen.
Unused solutions must be discarded after the time periods listed above.
Pharmacy Bulk Package: Aliquots of the reconstituted stock solution at 300 mg/mL are stable for up to 6 hours between 21° and 24°C (70° and 75°F) or up to 72 hours under refrigeration 4°C (40°F). The reconstituted stock solution should be held under refrigeration 4°C (40°F).
If the aliquots of the reconstituted stock solution (300 mg/mL) are held up to 6 hours between 21° and 24°C (70° and 75°F) or up to 72 hours under refrigeration 4°C (40°F) and further diluted to a concentration between 10 mg/mL and 100 mg/mL with any of the diluents listed below, then the following stability periods apply.
|
STABILITY PERIOD |
||
|
(31‑gram Pharmacy Bulk Package) |
||
|
Intravenous Solution (ticarcillin concentrations of 10 mg/mL to 100 mg/mL) |
Room Temperature 21° to 24°C (70° to 75°F) |
Refrigerated 4°C (40°F) |
|
Dextrose Injection 5%, USP |
24 hours |
3 days |
|
Sodium Chloride Injection 0.9%, USP |
24 hours |
4 days |
|
Lactated Ringer’s Injection, USP |
24 hours |
4 days |
|
Sterile Water for Injection, USP |
24 hours |
4 days |
If an aliquot of concentrated stock solution (300 mg/mL) is stored for up to 6 hours between 21° and 24°C (70° and 75°F) and then further diluted to a concentration between 10 mg/mL and 100 mg/mL, solutions of Sodium Chloride Injection, USP, Lactated Ringer’s Injection, USP, and Sterile Water for Injection, USP, may be stored frozen –18°C (0°F) for up to 30 days. Solutions prepared with Dextrose Injection 5%, USP, may be stored frozen –18°C (0°F) for up to 7 days. All thawed solutions should be used within 8 hours or discarded. Once thawed, solutions should not be refrozen.
Unused solutions must be discarded after the time periods listed above.
GALAXY Container (PL 2040 Plastic): Do not add supplementary medication to the container. The thawed solution is stable for 24 hours at room temperature 22°C (72°F) or for 7 days under refrigeration at 4°C (39°F).
3 DOSAGE FORMS AND STRENGTHS
The 3.1‑gram glass vial of TIMENTIN for Injection is a white to pale yellow sterile powder for reconstitution containing ticarcillin disodium equivalent to 3 grams ticarcillin and clavulanate potassium equivalent to 0.1 gram clavulanic acid.
The 31‑gram Pharmacy Bulk Package of TIMENTIN for Injection is a white to pale yellow sterile powder for reconstitution containing ticarcillin disodium equivalent to 30 grams ticarcillin and clavulanate potassium equivalent to 1 gram clavulanic acid.
The 100-mL single-dose GALAXY Container (PL 2040 Plastic) of TIMENTIN is a frozen solution containing ticarcillin disodium equivalent to 3.0 grams ticarcillin and clavulanate potassium equivalent to 0.1 gram clavulanic acid.
4 CONTRAINDICATIONS
TIMENTIN is contraindicated in patients who have a history of hypersensitivity reaction (e.g., anaphylaxis or erythema multiforme) to TIMENTIN or to other β‑lactam antibacterials (e.g., penicillins and cephalosporins).
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylactic Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam antibacterials. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. Before initiating therapy with TIMENTIN, inquire about previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, discontinue TIMENTIN and institute appropriate therapy.
5.2 Clostridium difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including TIMENTIN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Convulsions
Patients may experience convulsions when the dose of TIMENTIN exceeds the recommended dose, especially in the presence of impaired renal function [see Adverse Reactions (6.2), Overdosage (10)].
5.4 Risk of Bleeding
Some patients receiving β-lactam antibacterials have experienced bleeding associated with abnormalities in coagulation tests. These adverse reactions are more likely to occur in patients with renal impairment. If bleeding manifestations appear, treatment with TIMENTIN should be discontinued and appropriate therapy instituted.
5.5 Potential for Microbial Overgrowth or Bacterial Resistance
The possibility of superinfections with fungal or bacterial pathogens should be considered during therapy. If superinfections occur, appropriate measures should be taken.
5.6 Development of Drug-Resistant Bacteria
Prescribing TIMENTIN in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug‑resistant bacteria.
5.7 Interference with Laboratory Tests
High urine concentrations of ticarcillin may produce false-positive protein reactions (pseudoproteinuria) [see Drug Interactions (7.4)].
Clavulanic acid may cause a nonspecific binding of IgG and albumin by red cell membranes, leading to a false-positive Coombs test [see Drug Interactions (7.4)].
5.8 Electrolyte Imbalance
Hypokalemia has been reported during treatment with TIMENTIN. Serum potassium should be monitored in patients with fluid and electrolyte imbalance and in patients receiving prolonged therapy. The theoretical sodium content is 4.51 mEq (103.6 mg) per gram of TIMENTIN. This should be considered when treating patients requiring restricted salt intake.
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- •
- Anaphylactic Reactions [see Warnings and Precautions (5.1)]
- •
- Clostridium difficile Associated Diarrhea [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions occurring in ≥1% of 867 patients receiving TIMENTIN 3.1 grams in clinical studies included rash, nausea, diarrhea, and phlebitis at the injection site. The most common laboratory abnormalities (≥3%) were elevations in eosinophils, serum aspartate aminotransferase (AST), and serum alanine aminotransferase (ALT).
Available safety data for pediatric patients treated with TIMENTIN demonstrate a similar adverse event profile to that observed in adult patients.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postmarketing use of TIMENTIN. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These adverse reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to TIMENTIN.
Hypersensitivity Reactions: Skin rash, pruritus, urticaria, arthralgia, myalgia, drug fever, chills, chest discomfort, anaphylactic reactions, and bullous reactions (including erythema multiforme, toxic epidermal necrolysis, and Stevens‑Johnson syndrome).
Central Nervous System: Headache, giddiness, neuromuscular hyperirritability, or convulsive seizures.
Gastrointestinal Disturbances: Disturbances of taste and smell, stomatitis, flatulence, nausea, vomiting and diarrhea, epigastric pain, and pseudomembranous colitis have been reported. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment [see Warnings and Precautions (5.2)].
Hemic and Lymphatic Systems: Thrombocytopenia, leukopenia, neutropenia, eosinophilia, reduction of hemoglobin or hematocrit, and prolongation of prothrombin time and bleeding time.
Abnormalities of Hepatic Function Tests: Elevation of AST, ALT, serum alkaline phosphatase, serum LDH, and serum bilirubin. There have been reports of transient hepatitis and cholestatic jaundice, as with some other penicillins and some cephalosporins.
Renal and Urinary Effects: Hemorrhagic cystitis, elevation of serum creatinine and/or BUN, hypernatremia, reduction in serum potassium, and uric acid.
Local Reactions: Pain, burning, swelling, and induration at the injection site and thrombophlebitis with intravenous administration.
7 DRUG INTERACTIONS
7.1 Aminoglycosides
The mixing of TIMENTIN with an aminoglycoside in solutions for parenteral administration can result in substantial inactivation of the aminoglycoside.
7.2 Probenecid
Probenecid interferes with the renal tubular secretion of ticarcillin, thereby increasing serum concentrations and prolonging serum half‑life of ticarcillin. Probenecid does not affect the serum levels of clavulanic acid.
7.3 Oral Contraceptives
Ticarcillin disodium/clavulanate potassium may affect the gut flora, leading to lower estrogen reabsorption and reduced efficacy of combined oral estrogen/progesterone contraceptives.
7.4 Effects on Laboratory Tests
High urine concentrations of ticarcillin may produce false‑positive protein reactions (pseudoproteinuria) with certain methods. The bromphenol blue reagent strip test has been reported to be a reliable method for testing protein reactions [see Warnings and Precautions (5.7)].
Clavulanic acid in TIMENTIN may cause a nonspecific binding of IgG and albumin by red cell membranes, leading to a false‑positive Coombs test. A positive Coombs test should be interpreted with caution during treatment with TIMENTIN [see Warnings and Precautions (5.7)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B.
Reproduction studies have been performed in rats given doses up to 1,050 mg/kg/day (approximately half of the recommended human dose based on body surface area) and have revealed no evidence of harm to the fetus due to TIMENTIN. There are, however, no adequate and well‑controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether ticarcillin or clavulanic acid is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TIMENTIN is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of TIMENTIN have been established in the age group of 3 months to 16 years. Use of TIMENTIN in these age groups is supported by evidence from adequate and well‑controlled studies of TIMENTIN in adults with additional efficacy, safety, and pharmacokinetic data from both comparative and non‑comparative studies in pediatric patients. There are insufficient data to support the use of TIMENTIN in pediatric patients under 3 months of age.
If meningitis is suspected or documented, an alternative agent with demonstrated clinical efficacy in this setting should be used.
8.5 Geriatric Use
An analysis of clinical studies of TIMENTIN was conducted to determine whether subjects aged 65 and older respond differently from younger subjects. Of the 1,078 subjects treated with at least one dose of TIMENTIN, 67.5% were <65 years old, and 32.5% were ≥65 years old. No overall differences in safety or efficacy were observed between older and younger subjects, and other reported clinical experience have not identified differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.3)].
TIMENTIN contains 103.6 mg (4.51 mEq) of sodium per gram of TIMENTIN. At the usual recommended doses, patients would receive between 1,285 and 1,927 mg/day (56 and 84 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
10 OVERDOSAGE
In case of overdosage, discontinue TIMENTIN, treat symptomatically, and institute supportive measures as required. Ticarcillin and clavulanic acid may be removed from circulation by hemodialysis.
11 DESCRIPTION
TIMENTIN (ticarcillin disodium and clavulanate potassium) for Injection, 3.1‑gram glass vial, 31‑gram Pharmacy Bulk Package, and TIMENTIN (ticarcillin disodium and clavulanate potassium) Injection in the GALAXY Container (PL 2040 Plastic) are a combination of ticarcillin disodium and the β‑lactamase inhibitor clavulanate potassium (the potassium salt of clavulanic acid) for intravenous administration. Ticarcillin is derived from the basic penicillin nucleus, 6‑amino‑penicillanic acid.
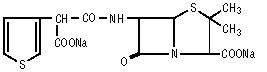
Chemically, ticarcillin disodium is N-(2-Carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-6-yl)-3-thiophenemalonamic acid disodium salt and may be represented as:
Clavulanic acid is produced by the fermentation of Streptomyces clavuligerus. It is a β‑lactam structurally related to the penicillins and possesses the ability to inactivate a wide variety of β‑lactamases by blocking the active sites of these enzymes. Clavulanic acid is particularly active against the clinically important plasmid‑mediated β‑lactamases frequently responsible for transferred drug resistance to penicillins and cephalosporins.
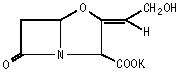
Chemically, clavulanate potassium is potassium (Z)-(2R,5R)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]heptane-2-carboxylate and may be represented structurally as:
TIMENTIN (ticarcillin disodium and clavulanate potassium) for Injection, the 3.1‑gram glass vial or the 31‑gram Pharmacy Bulk Package, are white to pale yellow sterile powders to be reconstituted and diluted for intravenous infusion. The reconstituted solution is clear, colorless or pale yellow, with a pH of 5.5 to 7.5. The 3.1‑gram glass vial of TIMENTIN for Injection contains ticarcillin disodium equivalent to 3 grams ticarcillin and clavulanate potassium equivalent to 0.1 gram clavulanic acid. The 31‑gram TIMENTIN for Injection Pharmacy Bulk Package contains ticarcillin disodium equivalent to 30 grams ticarcillin and clavulanate potassium equivalent to 1 gram clavulanic acid.
TIMENTIN (ticarcillin disodium and clavulanate potassium) Injection in the GALAXY Container (PL 2040 Plastic) is an iso‑osmotic, sterile, nonpyrogenic, frozen solution containing 3.0 grams ticarcillin as ticarcillin disodium and 0.1 gram clavulanic acid as clavulanate potassium. Approximately 0.3 gram sodium citrate hydrous, USP, is added as a buffer. Sodium hydroxide is used to adjust pH and convert ticarcillin monosodium to ticarcillin disodium. The pH may have been adjusted with hydrochloric acid. The solution is intended for intravenous use after thawing to room temperature. The pH of thawed solution ranges from 5.5 to 7.5.
The GALAXY container is fabricated from a specially designed multilayer plastic, PL 2040. Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
For the 3.1-gram dosage of TIMENTIN, the theoretical sodium content is 4.51 mEq (103.6 mg) per gram of TIMENTIN. The theoretical potassium content is 0.15 mEq (6 mg) per gram of TIMENTIN.
For the 3.1-gram dosage of TIMENTIN in the GALAXY Container, the theoretical total sodium content of the 100-mL solution is 18.7 mEq (429 mg), of which 15.6 mEq (359 mg) is contributed by the ticarcillin disodium component of TIMENTIN. The total theoretical potassium content of the 100-mL solution is 0.50 mEq (19.63 mg).
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption: After an intravenous infusion (30 minutes) of 3.1 grams of TIMENTIN, peak serum concentrations of both ticarcillin and clavulanic acid were attained immediately after completion of the infusion. Ticarcillin serum levels were similar to those produced by the administration of equivalent amounts of ticarcillin alone with a mean peak serum level of 324 mcg/mL. The corresponding mean peak serum level for clavulanic acid was 8 mcg/mL. (See Table 2.)
|
Time |
Ticarcillin Peak (Range) |
Clavulanic Acid Peak (Range) |
|
0 |
324 (293 ‑ 388) |
8.0 (5.3 ‑ 10.3) |
|
15 minutes |
223 (184 ‑ 293) |
4.6 (3.0 ‑ 7.6) |
|
30 minutes |
176 (135 ‑ 235) |
2.6 (1.8 ‑ 3.4) |
|
1 hour |
131 (102 ‑ 195) |
1.8 (1.6 ‑ 2.2) |
|
1.5 hours |
90 (65 ‑ 119) |
1.2 (0.8 ‑ 1.6) |
|
3.5 hours |
27 (19 ‑ 37) |
0.3 (0.2 ‑ 0.3) |
|
5.5 hours |
6 (5 ‑ 7) |
0 |
The mean area under the serum concentration curve was 485 mcg•hr/mL for ticarcillin and 8.2 mcg•hr/mL for clavulanic acid.
Distribution: Ticarcillin has been found to be approximately 45% bound to human serum protein and clavulanic acid approximately 25% bound. Ticarcillin can be detected in tissues and interstitial fluid following parenteral administration.
Distribution of ticarcillin into bile and pleural fluid has been demonstrated. The results of experiments involving the administration of clavulanic acid to animals suggest that this compound, like ticarcillin, is well distributed in body tissues.
Elimination: Approximately 60% to 70% of ticarcillin and approximately 35% to 45% of clavulanic acid are excreted unchanged in urine during the first 6 hours after administration of a single dose of TIMENTIN to normal volunteers with normal renal function. Two hours after an intravenous injection of 3.1 grams of TIMENTIN, concentrations of ticarcillin in urine generally exceed 1,500 mcg/mL. The corresponding concentrations of clavulanic acid in urine generally exceed 40 mcg/mL. By 4 to 6 hours after injection, the urine concentrations of ticarcillin and clavulanic acid usually decline to approximately 190 mcg/mL and 2 mcg/mL, respectively.
The mean serum half‑life of both ticarcillin and clavulanic acid in healthy volunteers was 1.1 hours.
Pediatrics: In pediatric patients receiving approximately 50 mg/kg of TIMENTIN (30:1 ratio ticarcillin to clavulanate), mean ticarcillin serum half‑lives were 4.4 hours in neonates (n = 18) and 1.0 hour in infants and children (n = 41). The corresponding clavulanate serum half‑lives averaged 1.9 hours in neonates (n = 14) and 0.9 hour in infants and children (n = 40). Area under the serum concentration time curves averaged 339 mcg•hr/mL in infants and children (n = 41), whereas the corresponding mean clavulanate area under the serum concentration time curves was approximately 7 mcg•hr/mL in the same population (n = 40).
Renal Impairment: An inverse relationship exists between the serum half‑life of ticarcillin and creatinine clearance. The half‑life of ticarcillin in patients with renal failure is approximately 13 hours. The dosage of TIMENTIN need only be adjusted in cases of severe renal impairment [see Dosage and Administration (2.3)].
Ticarcillin may be removed from patients undergoing dialysis; the actual amount removed depends on the duration and type of dialysis.
12.4 Microbiology
Mechanism of Action: Ticarcillin disrupts bacterial cell wall development by inhibiting peptidoglycan synthesis and/or by interacting with penicillin‑binding proteins.
Ticarcillin is susceptible to degradation by β‑lactamases, so the spectrum of activity does not normally include organisms which produce these enzymes.
Clavulanic acid is a β‑lactam, structurally related to the penicillins, which inactivates some β‑lactamase enzymes that are commonly found in bacteria resistant to penicillins and cephalosporins. In particular, it has good activity against the clinically important plasmid‑mediated β‑lactamases frequently responsible for transferred drug resistance.
The formulation of ticarcillin with clavulanic acid in TIMENTIN protects ticarcillin from degradation by β‑lactamase enzymes, effectively extending the antibacterial spectrum of ticarcillin to include many bacteria normally resistant to ticarcillin and other β‑lactam antibacterials.
Interaction With Other Antimicrobials: In vitro synergism between TIMENTIN and gentamicin, tobramycin, or amikacin against multi-resistant isolates of Pseudomonas aeruginosa has been demonstrated.
Ticarcillin/clavulanic acid has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections [see Indications and Usage (1)].
Susceptibility to ticarcillin/clavulanic acid will vary with geography and time; local susceptibility data should be consulted, if available.
Gram-positive bacteria
Staphylococcus aureus (methicillin-susceptible isolates only)
Staphylococcus epidermidis (methicillin-susceptible isolates only)
Gram-negative bacteria
Citrobacter spp.a
Enterobacter spp.a
E. cloacaea
Escherichia colia
Haemophilus influenzaeb
Klebsiella spp.a
K. pneumoniaea
Pseudomonas spp.a
P. aeruginosaa
Serratia marcescensa
Anaerobic bacteria
Bacteroides fragilis group
Prevotella melaninogenicus
a Some extended spectrum β‑lactamase (ESBL)–producing isolates are resistant to ticarcillin/clavulanic acid. Most carbapenemase-producing isolates are resistant to ticarcillin/clavulanic acid.
b β‑lactamase‑negative, ampicillin‑resistant (BLNAR) isolates of H. influenzae must be considered resistant to ticarcillin/clavulanic acid.
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for ticarcillin/clavulanic acid. However, the efficacy of ticarcillin/clavulanic acid in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria
Staphylococcus saprophyticus (methicillin-susceptible isolates only)
Streptococcus agalactiae (Group B)
Streptococcus bovis
Streptococcus pneumoniae (penicillin-susceptible isolates only)
Streptococcus pyogenes
Streptococcus spp. viridans group (penicillin-susceptible isolates only)
Gram-negative bacteria
Moraxella catarrhalis
Pasteurella multocida
Anaerobic bacteria
Clostridium spp.
C. perfringens
C. difficile
C. sporogenes
C. ramosum
C. bifermentans
Eubacterium spp.
Fusobacterium spp.
F. nucleatum
F. necrophorum
Peptostreptococcus spp.
Veillonella spp.
Susceptibility Testing: When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment.
Dilution Techniques: Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method2,4 (broth and/or agar). The MIC values should be interpreted according to criteria provided in Table 3.
Diffusion Techniques: Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method.3,4 These procedures use paper disks impregnated with 85 mcg of ticarcillin/clavulanate potassium (75 mcg ticarcillin plus 10 mcg clavulanate potassium) to test the susceptibility of bacteria to ticarcillin/clavulanic acid. The disc diffusion interpretive criteria are provided in Table 3.
Anaerobic Techniques: For anaerobic bacteria, susceptibility to ticarcillin/clavulanic acid can be determined by standardized test methods.4,5 The MIC values obtained should be interpreted according to the criteria in Table 3.
|
Microorganism |
Minimum Inhibitory Concentration (mcg/mL) |
Disc Diffusion (Zone Diameter mm) |
||||
|
S |
I |
R |
S |
I |
R |
|
|
Anaerobes |
≤32/2 |
64/2 |
≥128/2 |
- |
- |
- |
|
Enterobacteriaceae |
≤16/2 |
32/2 ‑ 64/2 |
≥128/2 |
≥20 |
15 - 19 |
≤14 |
|
Pseudomonas aeruginosa |
≤16/2 |
32/2 - 64/2 |
≥128/2 |
≥24 |
16 - 23 |
≤15 |
Susceptibility of staphylococci to ticarcillin/clavulanate may be deduced by testing penicillin and either oxacillin or cefoxitin.4
A report of “Susceptible” indicates the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations at the infection site necessary to inhibit growth of the pathogen. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the bacterium is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug product is physiologically concentrated or in situations where a high dosage of the drug product can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control: Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individual performing the tests.2,3,4,5 Standard ticarcillin/clavulanic acid powder should provide the following range of MIC values noted in Table 4. For the diffusion technique using the 85 mcg of ticarcillin/clavulanate potassium (75 mcg ticarcillin plus 10 mcg clavulanate potassium), the criteria in Table 4 should be achieved.
|
QC Strain |
Broth MIC (mcg/mL) |
Zone Diameter (mm) |
Agar Dilution MIC (mcg/mL) |
|
Bacteroides thetaiotaomicron ATCC 29741 |
0.5/2 - 2/2 |
- |
0.5/2 - 2/2 |
|
Clostridium difficile ATCC 700057 |
- |
- |
16/2 - 64/2 |
|
Enterococcus faecalis ATCC 29212 |
16/2 - 64/2 |
- |
- |
|
Escherichia coli ATCC 25922 |
4/2 - 16/2 |
24 - 30 |
- |
|
Escherichia coli ATCC 35218 |
8/2 - 32/2 |
21 - 25 |
- |
|
Eubacterium lentum ATCC 43055 |
8/2 - 32/2 |
- |
16/2 - 64/2 |
|
Pseudomonas aeruginosa ATCC 27853 |
8/2 - 32/2 |
20 - 28 |
- |
|
Staphylococcus aureus ATCC 29213 |
0.5/2- 2/2 |
- |
- |
|
Staphylococcus aureus ATCC 25923 |
- |
29 - 37 |
- |
ATCC = American Type Culture Collection
MIC = Minimum Inhibitory Concentration
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long‑term studies in animals have not been performed to evaluate carcinogenic potential. Results from in vitro assays in bacteria (Ames tests), yeast, and human lymphocytes, and in vivo in mouse bone marrow (micronucleus test) indicate TIMENTIN is without genotoxic potential.
14 CLINICAL STUDIES
TIMENTIN has been studied in 296 pediatric patients (excluding neonates and infants less than 3 months) in 6 controlled clinical trials. The majority of patients studied had intra‑abdominal infections, and the primary comparator was clindamycin and gentamicin with or without ampicillin. At the end‑of‑therapy visit, comparable efficacy was reported in the trial arms using TIMENTIN and an appropriate comparator.
TIMENTIN was also evaluated in an additional 408 pediatric patients (excluding neonates and infants less than 3 months) in 3 uncontrolled US clinical trials. Patients had a broad range of presenting diagnoses including: Infections in bone and joint, skin and skin structure, lower respiratory tract, urinary tract, as well as intra‑abdominal and gynecologic infections. Patients received TIMENTIN, either 300 mg/kg/day (based on the ticarcillin component) divided every 4 hours for severe infection or 200 mg/kg/day (based on the ticarcillin component) divided every 6 hours for mild to moderate infections. Efficacy rates were comparable to those obtained in controlled trials.
The adverse event profile in these 704 pediatric patients treated with TIMENTIN was comparable to that seen in adult patients.
15 REFERENCES
- •
- Cockcroft, DW, et al. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41, 1976.
- •
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Ninth Edition. 2012. CLSI document M07-A9. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- •
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Eleventh Edition. 2012. CLSI document M02-A11. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- •
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fourth Informational Supplement. 2014. CLSI document M100-S24. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- •
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard – Eighth Edition. 2012. CLSI document M11-A8. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
16 HOW SUPPLIED/STORAGE AND HANDLING
Each 3.1‑gram vial of TIMENTIN for Injection contains sterile ticarcillin disodium equivalent to 3 grams ticarcillin and sterile clavulanate potassium equivalent to 0.1 gram clavulanic acid.
|
NDC 0029-6571-26 |
3.1‑gram Vial |
Each 31‑gram Pharmacy Bulk Package of TIMENTIN for Injection contains sterile ticarcillin disodium equivalent to 30 grams ticarcillin and sterile clavulanate potassium equivalent to 1 gram clavulanic acid.
|
NDC 0029-6579-21 |
31‑gram Pharmacy Bulk Package |
Each 100‑mL single-dose GALAXY Container (PL 2040 Plastic) of TIMENTIN Injection contains ticarcillin disodium equivalent to 3.0 grams ticarcillin and clavulanate potassium equivalent to 0.1 gram clavulanic acid.
|
NDC 0029-6575-31 |
100 mL GALAXY Container (PL 2040 Plastic) |
3.1-gram Vials and 31-gram Pharmacy Bulk Packages of TIMENTIN for Injection should be stored at or below 25°C (77°F).
GALAXY Containers (PL 2040 Plastic) of TIMENTIN Injection should be stored at or below -20°C (-4°F). Avoid unnecessary handling of containers.
Thawing of Plastic Containers: Thaw frozen container at room temperature 22°C (72°F) or in a refrigerator 4°C (39°F). [Do not force thaw by immersion in water baths or by microwave irradiation.] Check for minute leaks by squeezing container firmly. If leaks are detected discard solution as sterility may be impaired. Do not add supplementary medication.
The container should be visually inspected. Thawed solutions should not be used unless clear; solutions will be light to dark yellow in color. Components of the solution may precipitate in the frozen state and will dissolve upon reaching room temperature with little or no agitation. If, after visual inspection, the solution remains cloudy or if an insoluble precipitate is noted or if any seals or outlet ports are not intact, the container should be discarded.
The thawed solution is stable for 24 hours at room temperature 22°C (72°F) or for 7 days under refrigeration 4°C (39°F).
Do not refreeze.
17 PATIENT COUNSELING INFORMATION
Drug Resistance: Inform patients that antibacterial drugs, including TIMENTIN, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When TIMENTIN is prescribed to treat a bacterial infection, inform patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by TIMENTIN or other antibacterial drugs in the future.
Clostridium difficile Associated Diarrhea: Inform patients that diarrhea is a common problem caused by antibacterials, and it usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having taken their last dose of the antibacterial. If this occurs, advise patients to contact their physician as soon as possible.
Allergic Reactions: Inform patients that TIMENTIN contains a penicillin that can cause allergic reactions in some individuals [see Warnings and Precautions (5.1)].
TIMENTIN is a registered trademark of the GSK group of companies.
GALAXY is a registered trademark of Baxter International Inc.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2014, the GSK group of companies. All rights reserved.
TMN:25PI
Principal Display Panel
NDC 0029-6571-26
TIMENTIN®
STERILE TICARCILLIN DISODIUM AND CLAVULANATE POTASSIUM
3.1 grams
Equivalent to TICARCILLIN, 3 GRAMS, CLAVULANIC ACID, 100 MG INJECTION
Rx only
Intravenous Infusion
Store dry powder at room temperature 21o to 25oC (70o – 77oF) or below.
Vial contains sterile ticarcillin disodium equivalent to 3 grams ticarcillin, and sterile clavulanate potassium equivalent to 100 mg clavulanic acid.
Dosage: See accompanying folder for complete prescribing information.
Directions for use: Reconstitute with approximately 13 mL Sterile Water for Injection, USP. Each mL of solution contains approximately 200 mg ticarcillin and 6.7 mg of clavulanic acid. Store solution for no more than 6 hours at room temperature (70o – 77oF) or 72 hours under refrigeration (40oF). This aqueous solution may be further diluted to 100 mL. Select diluent from I.V. solutions listed in prescribing information. Do not exceed specified storage times.
GlaxoSmithKline
RTP, NC 27709
Made in England. Rev. 10/13
10000000121260
Principal Display Panel
NDC 0029-6579-21
TIMENTIN®
STERILE TICARCILLIN DISODIUM AND CLAVULANATE POTASSIUM
31 grams
Equivalent to TICARCILLIN, 30 GRAMS, CLAVULANIC ACID, 1 GRAM INJECTION
Rx only
PHARMACY BULK PACKAGE NOT FOR DIRECT INFUSION
Intravenous Infusion
Store dry powder at room temperature 21o to 25oC (70o to 77oF) or below.
Store reconstituted stock solution refrigerated 4oC (40oF).
Vial contains sterile ticarcillin disodium equivalent to 30 grams ticarcillin, and sterile clavulanate potassium equivalent to 1 gram clavulanic acid.
Dosage: See accompanying folder for complete prescribing information.
Directions for use: Add 76 mL of a recommended I.V. solution (see prescribing information) and shake well. The solution will contain approximately 300 mg/mL ticarcillin and 10 mg/mL clavulanic acid. Transfer recommended dosage and further dilute to a desired concentration between 10 mg/mL and 100 mg/mL. Aliquoting operations must be completed within four hours of reconstitution. Discard the reconstituted stock solution four hours after initial entry.
GlaxoSmithKline
RTP, NC 27709
Made in England.
Rev. 10/13
10000000121292
Principal Display Panel
NDC 0029-6571-31
TIMENTIN®
TICARCILLIN DISODIUM AND CLAVULANATE POTASSIUM INJECTION
3.1 grams
Rx only
Galaxy
Single-Dose Container
100 mL
Iso-osmotic
Code 2G3545
Sterile, Nonpyrogenic
Each 100 mL contains: ticarcillin disodium equivalent to 3 grams ticarcillin, clavulanate potassium equivalent to 0.1 gram clavulanic acid and 0.3 gram sodium citrate hydrous, USP, added as a buffer. pH adjusted with sodium hydroxide and may have been adjusted with hydrochloric acid. pH 5.5 to 7.5.
Dosage: Intravenously as directed by a physician. See package insert.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks by squeezing thawed bag firmly. If leaks are found, discard bag as sterility may be impaired. Do not use unless solution is clear. Store at or below -20oC (-4oF). DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thaw at room temperature 22oC (72oF) or under refrigeration 4oC (39oF). The thawed solution is stable for 24 hours at room temperature or for 7 days under refrigeration. Do not refreeze.
TIMENTIN is a registered trademark of GlaxoSmithKline.
GALAXY is a registered trademark of Baxter International Inc.
Manufactured for GlaxoSmithKline
Research Triangle Park, NC 27709
by Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in England
PL 2040 Plastic
07-34-71-853
Rev. 6/13
10000000117823
| TIMENTIN
ticarcillin disodium and clavulanate potassium injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TIMENTIN
ticarcillin disodium and clavulanate potassium injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TIMENTIN
ticarcillin disodium and clavulanate potassium injection, solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - GlaxoSmithKline LLC (167380711) |