PILOPINE HS - pilocarpine hydrochloride gel
Alcon Laboratories, Inc.
----------
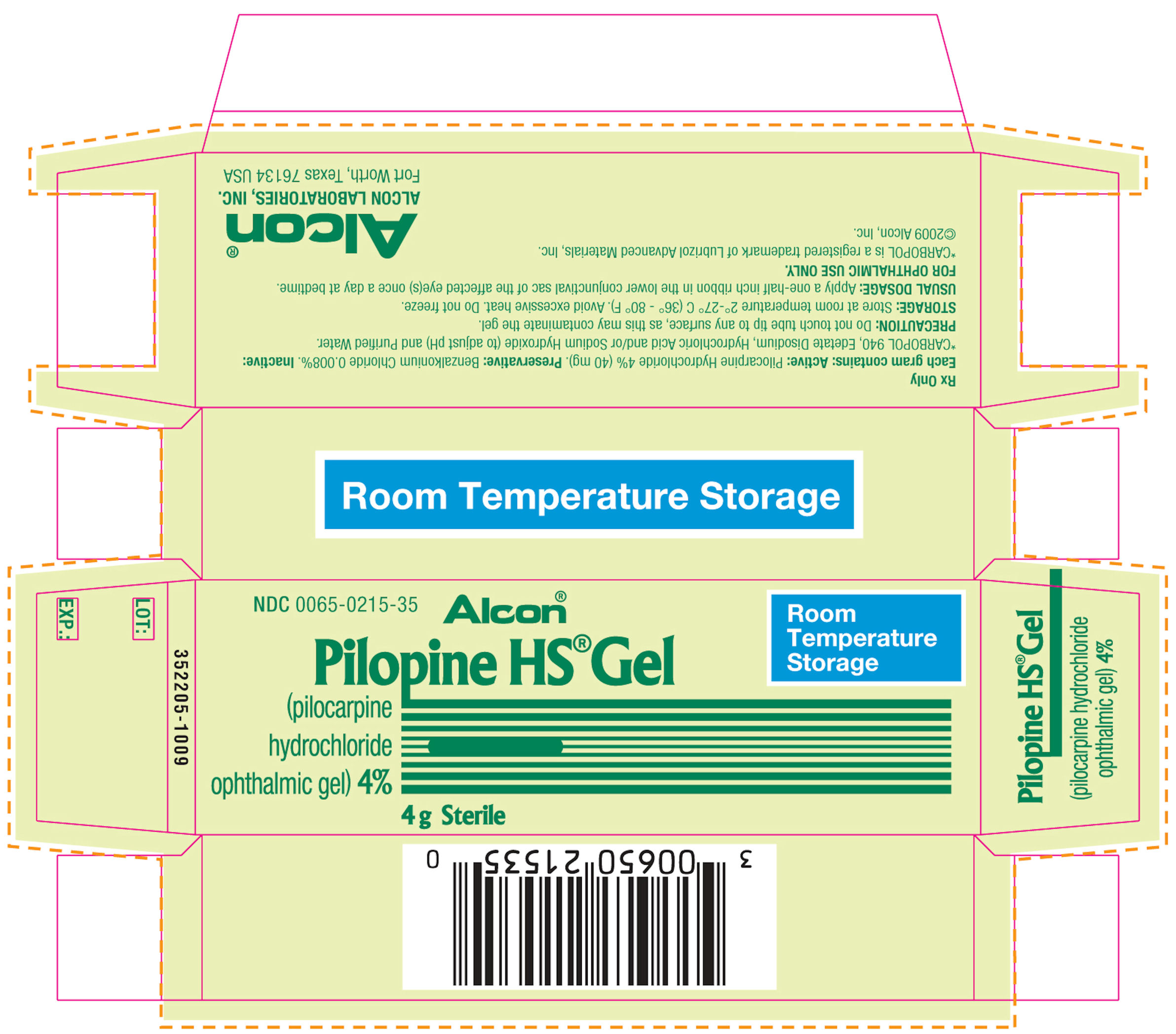
PILOPINE HS®
(pilocarpine hydrochloride ophthalmic gel) 4%
DESCRIPTION
PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4% is a sterile topical ophthalmic aqueous gel which contains more than 90% water and employs carbopol 940, a synthetic high molecular weight cross-linked polymer of acrylic acid, to impart a high viscosity. The active ingredient, pilocarpine hydrochloride, is a cholinergic agent and is represented by the chemical structure:
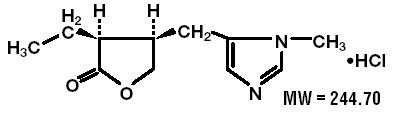
Established name: Pilocarpine Hydrochloride
Chemical name: 2(3H )-Furanone, 3-ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)-methyl]-, monohydrochloride, (3S-cis)-.
PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4% - Each Gram Contains: Active: pilocarpine hydrochloride 4% (40 mg). Preservative: benzalkonium chloride 0.008%. Inactives: carbopol 940, edetate disodium, hydrochloric acid and/or sodium hydroxide (to adjust pH) and purified water.
CLINICAL PHARMACOLOGY
PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4% is a direct acting cholinergic parasympathomimetic agent which acts through direct stimulation of muscarinic neuroreceptors and smooth muscle such as the iris and secretory glands. Pilocarpine produces miosis through contraction of the iris sphincter, causing increased tension on the scleral spur and opening of the trabecular meshwork spaces to facilitate outflow of aqueous humor. Outflow resistance is thereby reduced, lowering intraocular pressure.
INDICATIONS AND USAGE
PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4% is a miotic (parasympathomimetic) used to control intraocular pressure. It may be used in combination with other miotics, beta-blockers, carbonic anhydrase inhibitors, sympathomimetics or hyperosmotic agents.
CONTRAINDICATIONS
Miotics are contraindicated where constriction is undesirable, such as in acute iritis, and in those persons showing hypersensitivity to any of their components.
PRECAUTIONS
General
The miosis usually causes difficulty in dark adaptation. Patient should be advised to exercise caution in night driving and other hazardous occupations in poor illumination.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no long-term studies done using pilocarpine hydrochloride in animals to evaluate carcinogenic potential.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with pilocarpine hydrochloride. It is also not known whether pilocarpine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4% should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when pilocarpine hydrochloride is administered to a nursing woman.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
ADVERSE REACTIONS
The following adverse experiences associated with pilocarpine therapy have been reported: lacrimation, burning or discomfort, temporal or periorbital headache, ciliary spasm, conjunctival vascular congestion, superficial keratitis and induced myopia. Systemic reactions following topical administration are extremely rare, but occasional patients are peculiarly sensitive to develop sweating and gastrointestinal overactivity following suggested dosage and administration. Ocular reactions usually occur during initiation of therapy and often will not persist with continued therapy. Reduced visual acuity in poor illumination is frequently experienced in older individuals and in those with lens opacity. A subtle corneal granularity was observed in about 10% of patients treated with PILOPINE HS®. Cases of retinal detachment have been reported during treatment with miotic agents; especially in young myopic patients. Lens opacity may occur with prolonged use of pilocarpine.
OVERDOSAGE
Overdosage can produce sweating, salivation, nausea, tremors, and slowing of the pulse and a decrease in blood pressure. Bronchial constriction may develop in asthmatic patients. In moderate overdosage, spontaneous recovery is to be expected and is aided by intravenous fluids to compensate for dehydration. For cases demonstrating severe poisoning, atropine is the pharmacologic antagonist to pilocarpine.
A topical ocular overdose of an ophthalmic product containing pilocarpine may be flushed from the eye(s) with warm tap water.
DOSAGE AND ADMINISTRATION
Apply a one-half inch ribbon in the lower conjunctival sac of the affected eye(s) once a day at bedtime.
HOW SUPPLIED
PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4% is supplied as a 4% sterile aqueous gel in 4 gram tubes with ophthalmic tip.
4 gram: NDC 0065-0215-35
STORAGE: Store at room temperature 2°-27°C (36° -80° F). Avoid excessive heat. Do not freeze.
Rx Only
©2002, 2005, 2007, 2008 Alcon, Inc.
Revised: January 2008
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
How to use PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4%
PILOPINE HS® DOES NOT NEED TO BE REFRIGERATED. ENTIRE CONTENTS OF THIS TUBE MAY BE USED UP TO THE EXPIRATION DATE WHICH IS STAMPED ON THE CRIMP (BOTTOM) OF THE TUBE.
PILOPINE HS® (pilocarpine hydrochloride ophthalmic gel) 4% is an eye pressure lowering medication. The pilocarpine is absorbed into the eye at night to control intraocular pressure throughout the day. Apply about a half-inch ribbon (try not to put in too much) in the affected eye(s) every night just before going to bed. Should you occasionally apply too much and awaken with some gel on the eyelids, simply wash it away with warm water. You may initially experience some difficulty applying the gel or mild pilocarpine symptoms such as blurred vision in the morning. This is normal and you should soon become accustomed to both. If discomfort persists, consult your ophthalmologist. If you use other glaucoma medication at bedtime, use the drops first and wait at least five minutes before applying PILOPINE HS®.
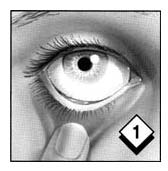
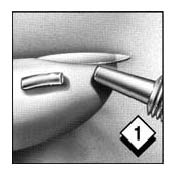
Wash and dry your hands. Remove cap from the tube and place it on a clean surface nearby. Gently pull down the lower eyelid to form a pocket for the gel.

Gently squeeze tube and apply a continuous strip (about a half-inch) of gel into and along the pocket. A half-inch is approximately as wide as your iris (the colored part of your eye).
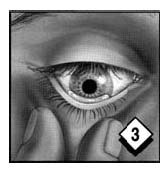
Gently grasp lower eyelid below lashes; lift lower eyelid and look downward as you close your eye. This will help move the gel deep into the pocket you have formed.
Keep eye closed for a minute or so to prevent excess blinking or tearing which might dilute the medication. Repeat the procedure for your other eye. Replace cap.
Shown below is another approach you can use if your ophthalmologist recommends working up to the full daily dosage.
EASY "FINGERTIP" APPLICATION
Start with a small amount of gel. As you become more familiar with the technique, you will be able to apply larger strips until you reach the half-inch maximum dose. Do not use too much gel. Individual situations vary, so be sure to follow your ophthalmologist's recommendations.
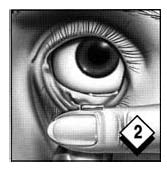
Wash hands thoroughly. Apply the correct amount of PILOPINE HS® to the edge of your index finger.
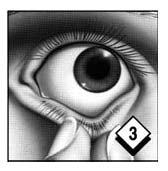
Pull down lower eyelid with middle finger of other hand to form a pouch. Gently roll the strip of gel into the eye pouch.
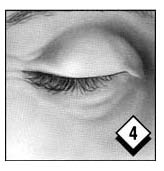
Gently squeeze the lower eyelid and pull it slightly away from your face.
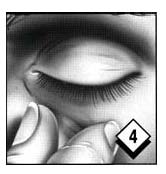
Look down and then close your eye for a moment. Wipe off any excess gel with a tissue. Repeat in the other eye if so directed by your ophthalmologist. Replace cap.
341175-0508
| PILOPINE HS
pilocarpine hydrochloride gel |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Registrant - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcon Research Ltd | 007672236 | MANUFACTURE(0065-0215) | |