DUKAL STERILE ALCOHOL SWABSTICK- isopropyl alcohol swab
Dukal Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Stop Use
If irritation and redness develop. If condition persists, consult your health care practitioner.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.

Dukal Corporation
Ref 887
NDC 65517-2022-1
Sterile
ALCOHOL
SWABSTICKS
Saturated with 70% Isopropyl Alcohol
For External Use Only
Sterility guaranteed unless package is damaged or opened.
1 / Pouch Latex 2
Manufactured For:
Dukal Corporation 631-656-3800
Ronkonkoma, NY 11779 www.dukal.com
Made in China
 Dukal Corporation
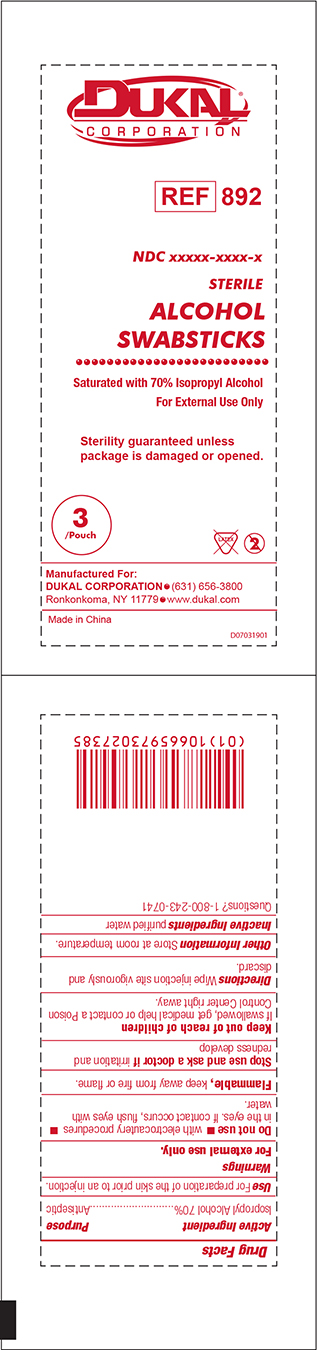
Dukal Corporation
Ref 892
NDC 65517-2022-3
Sterile
ALCOHOL
SWABSTICKS
Saturated with 70% Isopropyl Alcohol
For External Use Only
Sterility guaranteed unless package is damaged or opened.
3 / Pouch Latex 2
Manufactured For:
Dukal Corporation 631-656-3800
Ronkonkoma, NY 11779 www.dukal.com
Made in China
| DUKAL STERILE ALCOHOL SWABSTICK
isopropyl alcohol swab |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Dukal Corporation (791014871) |