Label: C-NATE DHA- omega-3 fatty acids, icosapent, doconexent, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, .alpha.-tocopherol, d-, cupric sulfate, zinc oxide, ferrous fumarate and magnesium oxide capsule, gelatin coated
- NDC Code(s): 23359-105-01, 23359-105-05, 23359-105-30
- Packager: Centurion Labs, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Description:
-
DESCRIPTION
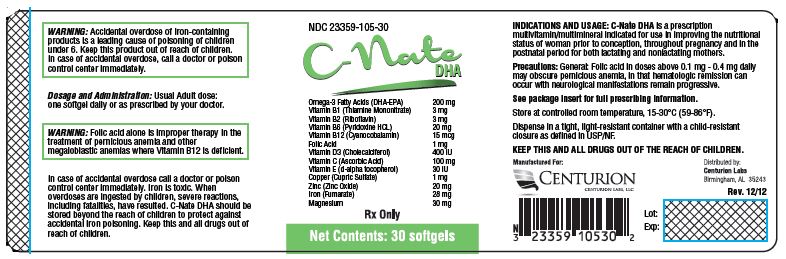
Each Softgel Contains: Omega-3 Fatty Acids (DHA-EPA) 200mg Vitamin B 1 (Thiamine Mononitrate) 3 mg Vitamin B 2 (Riboflavin) 3 mg Vitamin B6 (Pyridoxine HCL) 20 mg Vitamin B 12 (Cyanocobalamin) 15 mcg Folic Acid 1 mg Vitamin D 3 (Cholecalciferol) 400 IU Vitamin C (Ascorbic Acid) 100 mg Vitamin E (d-alpha tocopherol) 30 IU Copper (Cupric Sulfate) 1 mg Zinc (Zinc Oxide) 20 mg Iron (Fumerate) 28 mg Magnesium 30 mg - INACTIVE INGREDIENT
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
PRECAUTIONS: Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B 12 is deficient. Folic Acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
- WARNINGS
- PEDIATRIC USE
- GERIATRIC USE
- DRUG INTERACTIONS
-
ADVERSE REACTIONS
ADVERSE REACTIONS: Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 23359-105-30
C-Nate
DHASoftgels Omega-3 Fatty Acids (DHA-EPA) 200mg Vitamin B 1 (Thiamine Mononitrate) 3 mg Vitamin B 2 (Riboflavin) 3 mg Vitamin B 6 (Pyridoxine HCL) 20 mg Vitamin B 12 (Cyanocobalamin) 15 mcg Folic Acid 1 mg Vitamin D 3 (Cholecalciferol) 400 IU Vitamin C (Ascorbic Acid) 100 mg Vitamin E (d-alpha tocopherol) 30 IU Copper (Cupric Sulfate) 1 mg Zinc (Zinc Oxide) 20 mg Iron (Fumerate) 28 mg Magnesium 30 mg Rx Only
Net Contents: 30 softgels
Manufactured For:
Centurion Labs LLCDistributed by:
Centurion Labs LLC
Birmingham, AL 35243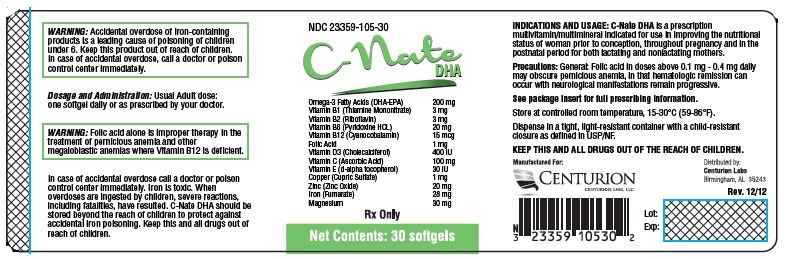
-
INGREDIENTS AND APPEARANCE
C-NATE DHA
omega-3 fatty acids, icosapent, doconexent, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, .alpha.-tocopherol, d-, cupric sulfate, zinc oxide, ferrous fumarate and magnesium oxide capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:23359-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) OMEGA-3 FATTY ACIDS 200 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 3 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 20 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 15 ug FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 100 mg .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 30 [iU] CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 20 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 28 mg MAGNESIUM (UNII: I38ZP9992A) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM 30 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) YELLOW WAX (UNII: 2ZA36H0S2V) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) WATER (UNII: 059QF0KO0R) Product Characteristics Color brown (annato) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code PRE;01 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23359-105-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2013 2 NDC:23359-105-05 5 in 1 CARTON; Type 0: Not a Combination Product 01/01/2013 3 NDC:23359-105-01 1 in 1 PACKET; Type 0: Not a Combination Product 01/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2013 Labeler - Centurion Labs, LLC (016481957)