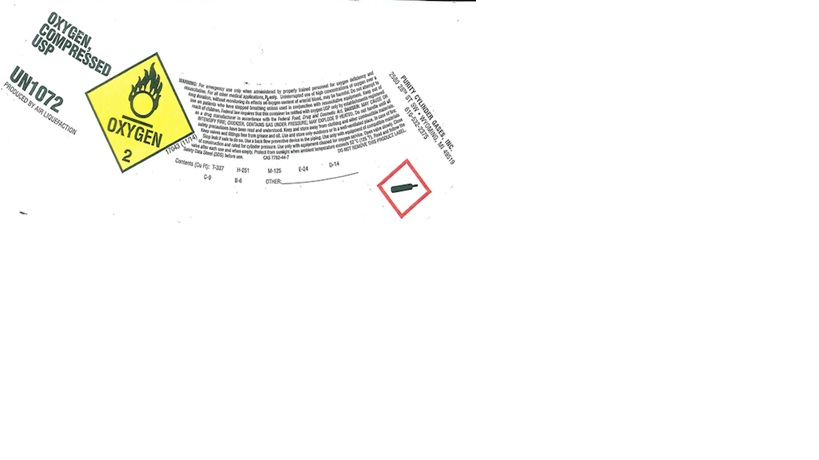
Label: OXYGEN gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 10927-103-09, 10927-103-10, 10927-103-11, 10927-103-12, view more10927-103-13, 10927-103-14, 10927-103-15, 10927-103-16, 10927-103-17, 10927-103-18, 10927-103-19 - Packager: PURITY CYLINDER GASES INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 27, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
OXYGEN, COMPRESSED USP
UN1072
PRODUCED BY AIR LIQUEFACTION
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and applications. For all other medical application, Rx only. Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Keep out of reach of children. Federal law requires that this container be refilled with oxygen USP only by establishment registered as a drug manufacturer in accordance with the Federal Food, Drug and Cosmetic Act. DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED. Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52 C (125 F). Read and follow the Safety Data Sheet (SDS) before use. CAS: 7782-44-7 DO NOT REMOVE THIS PRODUCT LABEL.
Contents (Cu Ft): T-337 H-251 M-125 E-24 D-14
C-9 B-6 OTHER:
PURITY CYLINDER GASES, INC.
2580 28TH ST SW, WYOMING, MI 49519
616-532-2375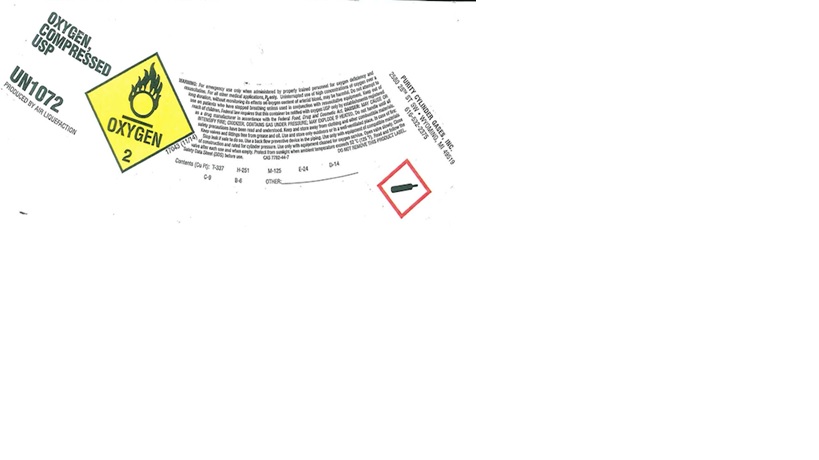
-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10927-103 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10927-103-09 113 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 2 NDC:10927-103-10 142 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 3 NDC:10927-103-11 255 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 4 NDC:10927-103-12 396 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 5 NDC:10927-103-13 651 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 6 NDC:10927-103-14 1388 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 7 NDC:10927-103-15 1699 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 8 NDC:10927-103-16 3597 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 9 NDC:10927-103-17 7420 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 10 NDC:10927-103-18 9657 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 11 NDC:10927-103-19 1132.68 L in 1 CYLINDER; Type 0: Not a Combination Product 10/20/1938 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205767 10/20/1938 Labeler - PURITY CYLINDER GASES INC (065878860) Establishment Name Address ID/FEI Business Operations PURITY CYLINDER GASES INC 006055710 manufacture(10927-103) Establishment Name Address ID/FEI Business Operations PURITY CYLINDER GASES INC 016676728 manufacture(10927-103) Establishment Name Address ID/FEI Business Operations PURITY CYLINDER GASES INC 120042374 manufacture(10927-103)