Label: PLIAGLIS- lidocaine and tetracaine cream
- NDC Code(s): 73575-009-02, 73575-009-04, 73575-009-09
- Packager: Oba Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PLIAGLIS safely and effectively. See full prescribing information for PLIAGLIS.
PLIAGLIS® (lidocaine and tetracaine) cream, for topical use
Initial U.S. Approval: 2006RECENT MAJOR CHANGES
INDICATIONS AND USAGE
PLIAGLIS is a combination of lidocaine, an amide local anesthetic, and tetracaine, an ester local anesthetic, indicated for use on intact skin in adults to provide topical local analgesia for superficial dermatological procedures such as dermal filler injection, pulsed dye laser therapy, facial laser resurfacing, and laser-assisted tattoo removal. (1)
DOSAGE AND ADMINISTRATION
- Apply only to intact skin. (2.1)
- Do not exceed the recommended dose of drug or duration of application. (2.1)
- Recommended duration of application (2.2):
For dermal filler injection ablative laser facial resurfacing, or pulsed-dye laser therapy 20-30 minutes prior to procedure For superficial dermatological procedures such as laser-assisted tattoo removal 60 minutes prior to procedure - See Full Prescribing Information for amount to apply based upon treatment site surface area. (2.3)
DOSAGE FORMS AND STRENGTHS
Cream: 70 mg of lidocaine and 70 mg of tetracaine per gram (7%; 7%). (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. (5.1)
-
Overexposure: To avoid overexposure that could lead to adverse effects,
- –
- do not use for longer duration or over larger surface areas than recommended. (5.2)
- –
- consider total amount of local anesthetics absorbed from all formulations. (5.2)
- –
- do not apply to mucous membranes or broken or inflamed skin. (5.2)
- –
- use with caution in patients who may be more sensitive to systemic effects of PLIAGLIS, including acutely ill or debilitated or those with severe hepatic disease or pseudocholinesterase deficiency. (5.2)
- Risk of Secondary Exposure to Children and Pets: Store and dispose of PLIAGLIS out of reach of children and pets due to the risk of accidental exposure and resulting toxicity. (5)
- Anaphylactic Reactions: Seek emergency help if an anaphylactic reaction occurs. (5.4)
- Eye Irritation: Avoid contact with eyes. (5.5)
ADVERSE REACTIONS
Most common local reactions were erythema (47%), skin discoloration (16%), and edema (14%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Taro Pharmaceuticals U.S.A., Inc. at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Antiarrhythmic Drugs: Use with caution in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) because the systemic toxic effects are thought to be additive and potentially synergistic with lidocaine and tetracaine. (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Recommended Dosing Duration
2.3 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Methemoglobinemia
5.2 Overexposure
5.3 Risks of Secondary Exposure to Children and Pets
5.4 Anaphylactic Reactions
5.5 Eye Irritation
5.6 Vaccinations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antiarrhythmic Drugs
7.2 Local Anesthetics
7.3 Drugs That May Cause Methemoglobinemia When Used with PLIAGLIS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- For use in adults only.
- PLIAGLIS should only be applied to intact skin.
- PLIAGLIS should not be applied to a procedure site after the procedure has been performed.
- Remove PLIAGLIS if skin irritation or a burning sensation occurs during application.
- In order to minimize the risk of systemic toxicity, do not exceed the recommended amount of drug to apply or the duration of the application [see Overdosage (10)].
- Avoid eye and lip contact with PLIAGLIS.
- Wash hands after handling PLIAGLIS.
- Upon removal from the treatment site, discard the used PLIAGLIS in a location that is out of the reach of children and pets. Access to PLIAGLIS by children or pets should be prevented during usage and storage of the product [see Warnings and Precautions (5.2)].
- Use only as directed.
2.2 Recommended Dosing Duration
- For superficial dermatological procedures, such as dermal filler injection, non-ablative laser facial resurfacing, or pulsed-dye laser therapy, apply PLIAGLIS to intact skin for 20 to 30 minutes prior to the procedure. See Table 1 for instructions on the amount to apply.
- For superficial dermatological procedures, such as laser-assisted tattoo removal, apply PLIAGLIS to intact skin for 60 minutes prior to the procedure. See Table 1 for instructions on the amount to apply.
2.3 Recommended Dosage
The dose of PLIAGLIS that provides effective local dermal analgesia depends on the duration of the application. Although not specifically studied, a shorter duration of application may result in a less complete dermal analgesia or a shorter duration of adequate dermal analgesia.
Determine the amount of drug to apply
The amount (length) of PLIAGLIS that should be dispensed is determined by the size of the area to be treated (see Table 1).
- Using the ruler on the applicator included in the carton, squeeze out and measure the amount of PLIAGLIS that approximates the amount required to achieve proper coverage.
- Spread PLIAGLIS evenly and thinly (approximately 1 mm or the thickness of a dime) across the treatment area using a flat-surfaced tool such as a metal spatula or tongue depressor.
- After waiting the required application time, remove the PLIAGLIS by grasping a free-edge with your fingers and pulling it away from the skin.
Table 1. Amount of PLIAGLIS According to Treatment Site Surface Area Surface Area of Treatment Site (inch2) Length of PLIAGLIS for 1 mm Thickness (inch) Weight of PLIAGLIS Dispensed (g) 2 1 1 3 2 3 6 5 5 12 9 11 16 12 13 23 18 20 31 24 26 39 30 33 47 36 40 54 42 46 62 48 53 - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- PLIAGLIS is contraindicated in patients with a known history of sensitivity to lidocaine or tetracaine, local anesthetics of the amide or ester type, or to any other component of the product [see Warnings and Precautions (5.4)].
- PLIAGLIS is contraindicated in patients with para-aminobenzoic acid (PABA) hypersensitivity.
-
5 WARNINGS AND PRECAUTIONS
5.1 Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue PLIAGLIS and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.2 Overexposure
- Application of PLIAGLIS for longer times than those recommended or application of PLIAGLIS over larger surface areas than those recommended could result in absorption of lidocaine and tetracaine at doses that could lead to serious adverse effects [see Overdosage (10)].
- When PLIAGLIS is used concomitantly with other products containing local anesthetic agents, consider the amount absorbed from all formulations since the systemic toxic effects are thought to be additive and potentially synergistic with lidocaine and tetracaine.
- PLIAGLIS is not recommended for use on mucous membranes or on areas with a compromised skin barrier because these uses have not been adequately studied. Application to broken or inflamed skin may result in toxic blood concentrations of lidocaine and tetracaine from increased absorption.
- Use PLIAGLIS with caution in patients who may be more sensitive to the systemic effects of lidocaine and tetracaine, including the acutely ill or debilitated.
- Patients with severe hepatic disease or pseudocholinesterase deficiency, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations of lidocaine and tetracaine.
5.3 Risks of Secondary Exposure to Children and Pets
Used PLIAGLIS contains a large amount of lidocaine and tetracaine. The potential exists for a small child or pet to suffer serious adverse effects from ingesting PLIAGLIS, although this risk with PLIAGLIS has not been evaluated. After use, replace the cap securely on the tube. It is important to store and dispose of PLIAGLIS out of the reach of children and pets.
5.4 Anaphylactic Reactions
Allergic or anaphylactic reactions have been associated with lidocaine and tetracaine and may occur with other components of PLIAGLIS. They are characterized by urticaria, angioedema, bronchospasm, and shock. If an allergic reaction occurs, seek emergency help immediately.
5.5 Eye Irritation
Avoid contact of PLIAGLIS with the eyes based on the findings of severe eye irritation with the use of similar products in animals. Also, the loss of protective reflexes may predispose to corneal irritation and potential abrasion. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Methemoglobinemia [see Warnings and Precautions (5.1)]
- Overexposure [see Warnings and Precautions (5.2)]
- Risks of Secondary Exposure in Children and Pets [see Warnings and Precautions (5.3)]
- Anaphylactic Reactions [see Warnings and Precautions (5.4)]
- Eye Irritation [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
However, the adverse reaction information from clinical trials does provide a basis for identifying the adverse events that appear to be related to drug use and for approximating their incidence in clinical practice.
PLIAGLIS has been evaluated for safety in 2159 persons undergoing a superficial dermal procedure. PLIAGLIS was studied in 11 placebo-controlled and 1 active-controlled trials, and in open-label safety trials. All 2159 persons were exposed to only a single application of PLIAGLIS. Adverse reactions were assessed by collecting spontaneously reported adverse reactions, and observations made on formal evaluation of the skin for specific reactions.
Most common adverse reactions in clinical trials
Localized Reactions: In clinical studies, the most common local reactions were erythema (47%), skin discoloration (e.g., blanching, ecchymosis, and purpura) (16%), and edema (14%). There were no serious adverse reactions. However, one patient withdrew due to burning pain at the treatment site.
Other Localized Reactions: The following dermal adverse reactions occurred in 1% or less of PLIAGLIS-treated patients: ecchymosis, petechial rash, vesiculobullous rash, perifollicular erythema, perifollicular edema, pruritus, rash, maculopapular rash, dry skin, contact dermatitis, and acne.
Systemic (Dose-Related) Reactions: Across all trials, 19 subjects experienced a systemic adverse reaction, 15 of whom were treated with PLIAGLIS and 4 with placebo. The frequency of systemic adverse reactions was greater for the PLIAGLIS group (1%) than the placebo group (0.3%). The most common systemic adverse events were headache, vomiting, dizziness, and fever, all of which occurred with a frequency of <1%. Other systemic reactions were syncope, nausea, confusion, dehydration, hyperventilation, hypotension, nervousness, paresthesia, pharyngitis, stupor, pallor, and sweating.
Systemic adverse reactions of lidocaine and tetracaine are similar in nature to those observed with other amide and ester local anesthetic agents, including CNS excitation and/or depression (lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensation of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest). Excitatory CNS reactions may be brief or not occur at all, in which case the first manifestation may be drowsiness merging into unconsciousness. Signs of CNS toxicity may start at plasma concentrations of lidocaine at 1000 ng/mL. The plasma concentrations at which tetracaine toxicity may occur are less well characterized; however, systemic toxicity with tetracaine is thought to occur with much lower plasma concentrations compared with lidocaine. The toxicity of co-administered local anesthetics is thought to be at least additive. Cardiovascular manifestations may include bradycardia, hypotension and cardiovascular collapse leading to arrest.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PLIAGLIS.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye disorders: Eyelid swelling
Skin: Pruritus, Rash, Skin Burning Sensation, Erythema, Urticaria
Other: Drug ineffective
-
7 DRUG INTERACTIONS
7.1 Antiarrhythmic Drugs
PLIAGLIS should be used with caution in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) since the systemic toxic effects are thought to be additive and potentially synergistic with lidocaine and tetracaine.
7.2 Local Anesthetics
When PLIAGLIS is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations should be considered since the systemic toxic effects are thought to be additive and potentially synergistic with lidocaine and tetracaine.
7.3 Drugs That May Cause Methemoglobinemia When Used with PLIAGLIS
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia:
Class Examples Nitrates/Nitrites nitric oxide, nitroglycerin, nitroprusside, nitrous oxide Local anesthetics articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine Antineoplastic agents cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase Antibiotics dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides Antimalarials chloroquine, primaquine Anticonvulsants phenobarbital, phenytoin, sodium valproate Other drugs acetaminophen, metoclopramide, quinine, sulfasalazine -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on PLIAGLIS use in pregnant women to determine a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Available data from an epidemiologic study and case series with parenteral lidocaine use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Published data on tetracaine use in pregnant women are not sufficient to determine any drug-associated risks. The amount of lidocaine and tetracaine systemically absorbed from PLIAGLIS is low compared to the parenteral route of administration and is not expected to result in significant fetal exposure. Systemic exposure of PLIAGLIS is directly related to both the duration of application and the surface area over which it is applied [see Clinical Pharmacology (12.3)].
In a published animal reproduction study, pregnant rats administered lidocaine by continuous subcutaneous infusion at doses approximately 1.3 times the maximum recommended human dose (MRHD) of 53 grams of PLIAGLIS during the period of organogenesis resulted in lower fetal body weights. In a published animal reproduction study, pregnant rats administered lidocaine, containing 1:100,000 epinephrine, injected into the masseter muscle of the jaw or into the gum of the lower jaw on Gestation Day 11 at 0.02 times the MRHD of PLIAGLIS resulted in developmental delays in neonates. Subcutaneous administration of tetracaine to pregnant rats and rabbits during organogenesis did not produce adverse embryofetal effects at 0.03 times the MRHD of PLIAGLIS (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Lidocaine administered by subcutaneous injection to pregnant rats during organogenesis was not teratogenic at doses up to 60 mg/kg (0.16 times the level of lidocaine contained in the MRHD of 53 grams of PLIAGLIS based on a mg/m2 body surface area (BSA) comparison). Lidocaine administered by subcutaneous injection to pregnant rabbits during organogenesis was not teratogenic at doses up to 15 mg/kg (0.08 times the level of lidocaine in the MRHD of PLIAGLIS on a mg/m2 basis).
In a published study, lidocaine administered to pregnant rats by continuous subcutaneous infusion during the period of organogenesis at 100, 250, and 500 mg/kg/day, did not produce any structural abnormalities, but did result in lower fetal weights at 500 mg/kg/day dose (approximately 1.3 times the level of lidocaine in the MRHD of PLIAGLIS on a mg/m2 basis) in the absence of maternal toxicity.
Tetracaine administered by subcutaneous injection to pregnant rats during organogenesis was not teratogenic at doses up to 10 mg/kg or in rabbits at doses up to 5 mg/kg (equivalent to 0.03 times of tetracaine in the MRHD of PLIAGLIS on a mg/m2 basis).
Lidocaine and tetracaine administered by subcutaneous injection to pregnant rats during organogenesis as a 1:1 eutectic mixture of 10 mg/kg each (equivalent to 0.03 times the active components in the MRHD of PLIAGLIS on a mg/m2 basis) was not teratogenic. Lidocaine and tetracaine by subcutaneous injection to pregnant rabbits during organogenesis as a 1:1 eutectic mixture of 5 mg/kg each was not teratogenic in rabbits (equivalent to 0.03 times the active components in the MRHD of PLIAGLIS on a mg/m2 basis).
Lidocaine containing 1:100,000 epinephrine at a dose of 6 mg/kg (approximately 0.02 times the level of lidocaine in the MRHD of PLIAGLIS on a mg/m2 basis) injected into the masseter muscle of the jaw or into the gum of the lower jaw of pregnant Long-Evans hooded rats on Gestation Day 11, lead to developmental delays in neonatal behavior among offspring. Developmental delays were observed for negative geotaxis, static righting reflex, visual discrimination response, sensitivity and response to thermal and electrical shock stimuli, and water maze acquisition. The developmental delays of the neonatal animals were transient with responses becoming comparable to untreated animals later in life. The clinical relevance of the animal data is uncertain.
Pre- and post-natal maturational, behavioral, or reproductive development was not affected by maternal subcutaneous administration of tetracaine during gestation (Gestation Day 6 to Post-Partum Day 20) and lactation up to doses of 7.5 mg/kg (equivalent to 0.02 times the level of tetracaine in the MRHD of PLIAGLIS on a mg/m2 basis).
8.2 Lactation
Risk Summary
There are no data on the presence of tetracaine in human milk, the effects on a breastfed infant, or the effects on milk production. Published studies on parenterally administered lidocaine have reported the presence of lidocaine in human milk with milk:plasma ratios ranging between 0.4 to 1.1. Available data on lidocaine's effects on the breastfed child have not revealed a consistent pattern of associated adverse effects. The amount of lidocaine present in human milk following topical administration is unknown, but systemic absorption from topical lidocaine is low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PLIAGLIS and any potential adverse effects on the breastfed child from PLIAGLIS or the mother's underlying condition.
8.4 Pediatric Use
Safety and effectiveness of PLIAGLIS in pediatric patients have not been established. Unintended exposure in pediatric patients could possibly lead to serious adverse effects [see Warnings and Precautions (5.2)]. In a trial of PLIAGLIS in pediatric patients aged 5 to 17 years undergoing venipuncture (blood draw or intravenous line placement), PLIAGLIS applied for 30 minutes failed to show efficacy over placebo in reducing the pain associated with the procedure.
8.5 Geriatric Use
Of the total number of subjects treated with PLIAGLIS in controlled clinical studies, 161 subjects were 65 years and older, while 50 subjects were over 75 years of age. No overall differences in safety and effectiveness were observed between these subjects and younger subjects. However, increased sensitivity in individual patients aged 65 years and older cannot be ruled out [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Application of 59 g of PLIAGLIS over 400 cm2 (62 inch2) for up to 120 minutes to adults produces peak plasma concentrations of lidocaine of 220 ng/mL. Toxic levels of lidocaine (>5000 ng/mL) cause CNS toxicity, including the risk of seizure. Signs of CNS toxicity may start at plasma concentrations of lidocaine as low as 1000 ng/mL, and the risk of seizures generally increases with increasing plasma levels. Very high levels of lidocaine can cause respiratory arrest, coma, decreases in cardiac output, total peripheral resistance and mean arterial pressure, ventricular arrhythmias and cardiac arrest.
Tetracaine is associated with a profile of systemic CNS and cardiovascular adverse events similar to lidocaine, although toxicity associated with tetracaine is thought to occur at lower doses compared to lidocaine. The toxicity of co-administered local anesthetics is thought to be at least additive. In the absence of massive topical overdose or oral ingestion, other etiologies for the clinical effects or overdosage from other sources of lidocaine, tetracaine or other local anesthetics should be considered.
The management of overdosage includes close monitoring, supportive care and symptomatic treatment. Dialysis is of negligible value in the treatment of acute overdosage of lidocaine or tetracaine.
-
11 DESCRIPTION
PLIAGLIS (lidocaine and tetracaine) Cream 7% / 7% is a topical local anesthetic cream that forms a pliable peel on the skin when exposed to air. The drug formulation is an emulsion in which the oil phase is a 1:1 eutectic mixture of lidocaine 7% and tetracaine 7%. The eutectic mixture has a melting point below room temperature and therefore both local anesthetics exist as a liquid oil rather than as crystals.
The net weight of lidocaine is 2.1 g and of tetracaine is 2.1 g per 30 g tube.
Lidocaine, an amide local anesthetic, is chemically designated as acetamide,2-(diethylamino)-N-(2,6-dimethylphenyl) and has an octanol:water partition ratio of 182 at pH 7.3.
The molecular weight of lidocaine is 234.3, and the molecular formula is C14H22N2O. The structural formula is:
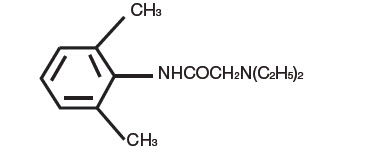
Tetracaine, an ester local anesthetic, is chemically designated as 2-dimethylaminoethyl 4-n-butyl-aminobenzoate and has an octanol:water partition ratio of 5370 at pH 7.3. The molecular weight of tetracaine is 264.4, and the molecular formula is C15H24N2O2. The structural formula is:
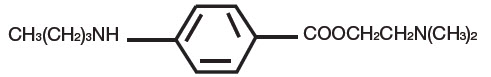
Each gram of PLIAGLIS contains lidocaine 70 mg and tetracaine 70 mg in a 1:1 eutectic mixture and it also contains the following inactive ingredients: anhydrous dibasic calcium phosphate, methylparaben, polyvinyl alcohol, propylparaben, purified water, sorbitan monopalmitate, and white petrolatum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lidocaine is an amide-type local anesthetic agent and tetracaine is an ester-type local anesthetic agent. Both lidocaine and tetracaine block sodium ion channels required for the initiation and conduction of neuronal impulses which, in certain instances, results in local anesthesia. When applied to intact skin, PLIAGLIS provides local dermal analgesia by the release of lidocaine and tetracaine from the peel into the skin.
12.2 Pharmacodynamics
Duration of analgesia was evaluated using a pinprick test in 40 adult volunteers. The median duration of analgesia was 11 hours. There was no difference between the 30-minute and 60-minute PLIAGLIS application periods with respect to the mean for time to return of sensation. However, 55% of PLIAGLIS treated subjects still reported diminished sensation at the end of the 13-hour study period.
12.3 Pharmacokinetics
Absorption: The amount of lidocaine and tetracaine systemically absorbed from PLIAGLIS is directly related to both the duration of application and the surface area over which it is applied, Table 2.
Application of 59 g of PLIAGLIS over 400 cm2 (62 inch2) for up to 120 minutes to adults produces peak plasma concentrations of lidocaine of 220 ng/mL. Tetracaine plasma levels were not measurable (<0.9 ng/mL). Systemic exposure to lidocaine, as measured by Cmax and AUC0-24, was proportional to the application area, and increased with application time up to 60 minutes.
Table 2. Absorption of lidocaine and tetracaine following application of PLIAGLIS PLIAGLIS Cream (g) Area
(cm2)Age Range
(yr)n Application Time (min) Drug Content
(g)Mean Cmax
(ng/mL)Mean Tmax
(hr)na=not applicable
400 cm2 = approximately 62 inch221 400 18-64 4 30 Lidocaine, 1.5 49 4.0 Tetracaine, 1.5 <0.9 na 33 400 18-64 4 60 Lidocaine, 2.3 96 2.8 Tetracaine, 2.3 <0.9 na 31 400 ≤ 65 6 60 Lidocaine, 2.2 48 3.8 Tetracaine, 2.2 <0.9 na Distribution: When lidocaine is administered intravenously to healthy volunteers, the steady-state volume of distribution is approximately 0.8 to 1.3 L/kg. At lidocaine concentrations observed following the recommended product application, approximately 75% of lidocaine is bound to plasma proteins, primarily alpha-1-acid glycoprotein. At much higher plasma concentrations (1 to 4 mg/mL of free base) the plasma protein binding of lidocaine is concentration dependent. Lidocaine crosses the placental and blood brain barriers, presumably by passive diffusion. CNS toxicity may typically be observed around 5000 ng/mL of lidocaine; however, a small number of patients reportedly may show signs of toxicity at approximately 1000 ng/mL [see Overdosage (10)]. Volume of distribution and protein binding have not been determined for tetracaine due to rapid hydrolysis in plasma.
Metabolism: It is not known if lidocaine or tetracaine is metabolized in the skin. Lidocaine is metabolized rapidly by the liver to a number of metabolites, including monoethylglycinexylidide (MEGX) and glycinexylidide (GX), both of which have pharmacologic activity similar to, but less potent than that of lidocaine. The major metabolic pathway of lidocaine, sequential N-deethylation to MEGX and GX, is primarily mediated by CYP1A2 with a minor role of CYP3A4. The metabolite, 2,6- xylidine, has unknown pharmacologic activity. Following intravenous administration of lidocaine, MEGX and GX concentrations in serum range from 11% to 36% and from 5% to 11% of lidocaine concentrations, respectively. Serum concentrations of MEGX were about one-third the serum lidocaine concentrations.
Tetracaine undergoes rapid hydrolysis by plasma esterases. Primary metabolites of tetracaine include para-aminobenzoic acid and diethylaminoethanol, both of which have an unspecified activity.
Elimination: The half-life of lidocaine elimination from the plasma following intravenous administration is approximately 1.8 hr. Lidocaine and its metabolites are excreted by the kidneys. More than 98% of an absorbed dose of lidocaine can be recovered in the urine as metabolites or parent drug. Less than 10% of lidocaine is excreted unchanged in adults, and approximately 20% is excreted unchanged in neonates. The systemic clearance is approximately 8 to 10 mL/min/kg. During intravenous studies, the elimination half-life of lidocaine was statistically significantly longer in elderly patients (2.5 hours) than in younger patients (1.5 hours). The half-life and clearance for tetracaine has not been established for humans, but hydrolysis in the plasma is rapid.
Special Populations
Elderly: After application of 31g of PLIAGLIS over 400 cm2 (62 inch2) for 60 minutes, mean peak plasma levels of lidocaine were 48 ng/mL for elderly patients (>65 years of age, mean 68.0 ± 3.2 years, n = 6). These levels are similar to or lower than those for younger patients receiving similar amounts of PLIAGLIS.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Long-term studies in animals have not been performed to evaluate the carcinogenic potential of either lidocaine or tetracaine.
Mutagenesis: The mutagenic potential of lidocaine base and tetracaine base has been determined in the in vitro Ames bacterial reverse mutation assay, the in vitro chromosome aberration assay using Chinese hamster ovary cells, and the in vivo mouse micronucleus assay. Lidocaine was negative in all three assays. Tetracaine was negative in the in vitro Ames assay and the in vivo mouse micronucleus assay. In the in vitro chromosome aberration assay, tetracaine was negative in the absence of metabolic activation, and equivocal in the presence of metabolic activation.
Impairment of Fertility: Lidocaine did not affect fertility in female rats when given via continuous subcutaneous infusion via osmotic minipumps up to doses of 250 mg/kg/day (0.66 times the level of lidocaine in the MRHD of PLIAGLIS based on a mg/m2 body surface area comparison). Lidocaine treatment did not affect overall fertility in male rats when given as subcutaneous doses up to 60 mg/kg (0.16 times the level of lidocaine contained in the MRHD of PLIAGLIS based on a mg/m2 basis), although the treatment caused an increased copulatory interval and led to a dose-related decrease in homogenization resistant sperm head count, daily sperm production, and spermatogenic efficiency. Tetracaine did not affect fertility in male or female rats when given as subcutaneous doses up to 7.5 mg/kg (equivalent to 0.04 times the level of tetracaine in the MRHD of PLIAGLIS on a mg/m2 basis).
-
14 CLINICAL STUDIES
In four clinical trials, adult patients were treated with PLIAGLIS or placebo prior to undergoing a superficial dermatologic procedure. Drug was applied for 20 or 30 minutes for dermatologic procedures such as dermal filler injection, pulsed dye laser therapy, and facial laser resurfacing. Drug was applied for 60 minutes for laser-assisted tattoo removal.
Treatment with PLIAGLIS resulted in statistically significantly less pain compared to placebo treatment, as measured by a 100-mm visual analog scale (VAS). Patient efficacy ratings are shown in Table 3.
Table 3. Summary of patient evaluations following application of PLIAGLIS and placebo Mean VAS score Dermatologic Procedure PLIAGLIS Cream Placebo 20 Min Application Pulsed Dye Laser Therapy (N=80) 16 31 30 Min Application Non-Ablative Laser Facial Resurfacing (N=54) 21 38 Dermal Filler Injections (N=70) 24 37 60 Min Application Laser-Assisted Tattoo Removal (N=62) 39 59 - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Prior to treatment, advise patient of the following:
- Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
- Advise patients to inform the healthcare provider if they experience skin irritation or a burning sensation because the product will need to be immediately removed [see Dosage and Administration (2.1)].
- Advise patients of the signs and symptoms of hypersensitivity reactions and to seek immediate medical attention should they occur [see Warnings and Precautions (5.4)].
- Advise patients that PLIAGLIS may lead to diminished or blocked sensation in the treated skin and to avoid inadvertent trauma through rubbing, scratching, or exposure to heat or cold before complete sensation occurs.
- Advise breastfeeding women not to apply PLIAGLIS directly to the nipple and areola to avoid direct infant exposure [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
Instructions for Use
Pliaglis®[Ply-uh-gliss]
(lidocaine and tetracaine) CreamRead and follow this Instructions for Use before applying PLIAGLIS Cream.
PLIAGLIS Cream is a prescription medicine used on adults to numb your skin to reduce the pain associated with medical procedures on the skin. After PLIAGLIS Cream is applied to your skin, it will form a film (or mask) that is removed before your procedure.
Important Information:
- Keep PLIAGLIS Cream out of the reach of children and away from pets.
- Do not apply PLIAGLIS Cream to skin that is broken, cut, scratched, irritated (red) or has a rash.
- Do not apply PLIAGLIS to a procedure site after the procedure has been performed.
- Do not use more PLIAGLIS Cream than prescribed.
- Do not leave PLIAGLIS Cream on your skin for longer than 120 minutes (2 hours).
- Avoid getting PLIAGLIS Cream in your eyes or on your lips. If contact occurs, rinse immediately with water and if irritation occurs, seek medical advice.
- Always wash your hands after handling PLIAGLIS Cream.
- Do not cover PLIAGLIS Cream with waterproof (occlusive) dressing or overdosage may occur.
- Store PLIAGLIS Cream in the refrigerator. PLIAGLIS Cream may be stored at room temperature for up to 3 months
Step 1: Check the skin where the cream will be applied.
- Check the skin area to be treated to make sure the skin is intact.
- Read and follow Important Information notices in grey box above.
Step 2: Estimate the amount of PLIAGLIS Cream to apply.
- Apply PLIAGLIS Cream exactly as your healthcare provider tells you to. Your healthcare provider will tell you the area to be treated, when to apply the cream, and how long to leave it on your skin.
- Estimate the area of the skin to be treated and the amount of PLIAGLIS Cream to apply using the table below and the ruler on the applicator included in the carton. Break the cream length into smaller segments that fit inside the treatment area.
Approximate size of area to be treated Length of cream (inches) based on 30-gram tube 1 inch × 1 inch ¾ inch 2 inches × 2 inches 3 inches 2 inches × 3 inches (size of a credit card) 5 inches 3 inches × 5 inches (size of a 3 inch × 5 inch photo) 12 inches (½ tube) 4 inches × 6 inches (size of a 4 inch × 6 inch postcard) 18 inches (⅔ tube) Half of your face 1 tube 6 inches × 8 inches (size of two 4 inch × 6 inch postcards) 1½ tubes Full face 2 tubes
Step 3: Open the tube and apply PLIAGLIS Cream to your skin.
- Open the tube by pushing down the cap while turning counter-clockwise.
- Using the table above and the ruler on the applicator included in the carton as a guide, squeeze out the amount of PLIAGLIS Cream needed for your prescribed dose directly onto your treatment area.
- Put the cap back on tightly.
- Using the applicator included in the carton, spread PLIAGLIS Cream in an even white layer, about the thickness of a dime, over the treatment area.
- If you get PLIAGLIS Cream on an area you do not intend to cover, wash the cream from that area.
- Do not rub PLIAGLIS Cream into your skin.
- Do not touch PLIAGLIS Cream after application. Let it air dry.
- Wash your hands.

open tube
spread cream
spread cream
wash hands
Step 4: Leave PLIAGLIS Cream on your skin for the recommended time.
- Your healthcare provider will tell you how long to leave PLIAGLIS Cream on your skin. Ask your healthcare provider if you are not sure how long to leave PLIAGLIS Cream on your skin.
- If skin irritation or a burning sensation happens after applying PLIAGLIS Cream, remove the PLIAGLIS Cream right away. Wash the cream off with soap and water. Tell your healthcare provider if your skin irritation does not go away.
Step 5: Remove and throw away (discard) the dried PLIAGLIS Cream.
- After leaving PLIAGLIS Cream on your skin the recommended amount of time, carefully peel the dried cream off your skin starting at the edges, wipe off any excess cream with a tissue or paper towel, and then throw it all away in the household trash.
- Wash your hands after removing or touching PLIAGLIS Cream.
- The treated area of your skin may be numb up to 9 hours.
-
Be careful with the treated skin area.
Do not scratch the treated skin area. Keep the treated skin area protected from extreme heat or cold until sensation returns.
remove cream
remove cream
discard cream
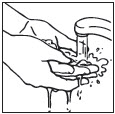
wash hands
Step 6: Clean and Store Applicator
- Wash the applicator with soap and water to remove any PLIAGLIS Cream remaining on the applicator.
- Dry the applicator.
- Store the applicator in the carton with the tube of PLIAGLIS Cream for future use.
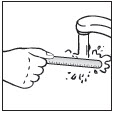
clean applicator
store applicator
This Instructions for Use has been approved by the U.S. Food and Drug Administration
Issued: 01/2020 - PRINCIPAL DISPLAY PANEL - 30 g Tube Carton
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PLIAGLIS
lidocaine and tetracaine creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73575-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lidocaine (UNII: 98PI200987) (lidocaine - UNII:98PI200987) lidocaine 70 mg in 1 g tetracaine (UNII: 0619F35CGV) (tetracaine - UNII:0619F35CGV) tetracaine 70 mg in 1 g Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) METHYLPARABEN (UNII: A2I8C7HI9T) PETROLATUM (UNII: 4T6H12BN9U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73575-009-09 1 in 1 CARTON 01/24/2020 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:73575-009-02 2 in 1 CARTON 01/24/2020 2 30 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:73575-009-04 4 in 1 CARTON 01/24/2020 3 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021717 01/24/2020 Labeler - Oba Pharmaceuticals, Inc. (117380833)






