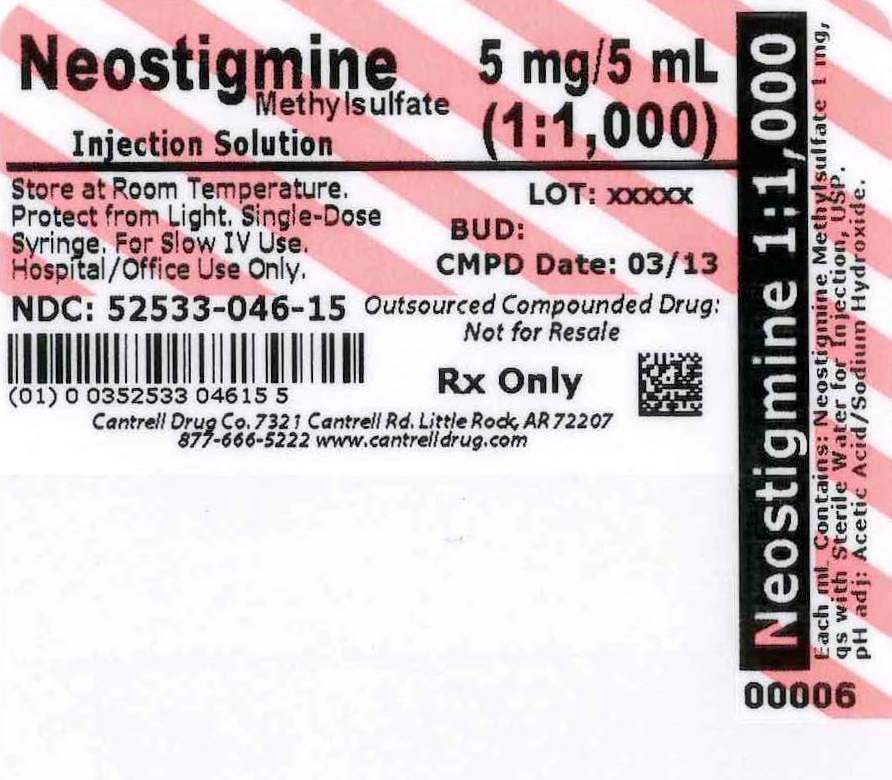
NEOSTIGMINE METHYLSULFATE- neostigmine methylsulfate injection, solution
Cantrell Drug Company
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Neostigmine Methylsulfate 1:1,000 Injection Solution 5 mL Syringe
• HOW SUPPLIED
Neostigmine Methylsulfate is supplied as a sterile, nonpyrogenic solution that is clear, colorless in a 5 mL Single Use Syringe.
This product is Preservative-Free and Latex-Free.
• INGREDIENTS
Each 1 mL contains 1 mg neostigmine methylsulfate in Water for Injection q.s. The aqueous solution is adjusted to a pH of 5.0- 6.5 with acetic acid and/or sodium hydroxide, if necessary.
• STORAGE AND HANDLING
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]. Protect from light.
| NEOSTIGMINE METHYLSULFATE
neostigmine methylsulfate injection, solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Cantrell Drug Company (035545763) |
Revised: 12/2017
Document Id: 5fae791e-af67-3bdc-e053-2a91aa0ae801
Set id: 86963fc9-468c-4842-a76a-c5c1f5510c75
Version: 2
Effective Time: 20171206
Cantrell Drug Company