TECHNIVIE- ombitasvir and paritaprevir and ritonavir
AbbVie Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TECHNIVIE safely and effectively. See full prescribing information for TECHNIVIE.
TECHNIVIE (ombitasvir, paritaprevir and ritonavir) tablets, for oral use Initial U.S. Approval: 2015 WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBVSee full prescribing information for complete boxed warning.Hepatitis B virus (HBV) reactivation has been reported, in some cases resulting in fulminant hepatitis, hepatic failure, and death. (5.1) RECENT MAJOR CHANGES
INDICATIONS AND USAGETECHNIVIE is a fixed-dose combination of ombitasvir, a hepatitis C virus NS5A inhibitor, paritaprevir, a hepatitis C virus NS3/4A protease inhibitor, and ritonavir, a CYP3A inhibitor and is indicated in combination with ribavirin for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis. (1) DOSAGE AND ADMINISTRATIONTesting Prior to the Initiation of Therapy:
DOSAGE FORMS AND STRENGTHSTablets: 12.5 mg ombitasvir, 75 mg paritaprevir, 50 mg ritonavir. (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most commonly reported adverse reactions (incidence greater than 10% of subjects, all grades) observed with treatment with ombitasvir, paritaprevir and ritonavir with ribavirin for 12 weeks in patients without cirrhosis were asthenia, fatigue, nausea and insomnia. (6.1) The most common adverse events (incidence greater than 10% of subjects, all grades) observed with treatment with TECHNIVIE and ribavirin for 12 weeks in patients in compensated cirrhosis were fatigue, asthenia, headache, musculoskeletal pain, pruritus, insomnia/sleep disorder, skin reactions, mood disorders, nausea, dizziness and dyspnea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 12/2019 |
||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
Test all patients for evidence of current or prior hepatitis B virus (HBV) infection before initiating treatment with TECHNIVIE. HBV reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals and were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Monitor HCV/HBV coinfected patients for hepatitis flare or HBV reactivation during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
TECHNIVIE is indicated in combination with ribavirin for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to the Initiation of Therapy
- Test all patients for evidence of current or prior HBV infection by measuring hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc) before initiating HCV treatment with TECHNIVIE [see Warnings and Precautions (5.1)].
- Prior to initiation of TECHNIVIE, assess hepatic laboratory and clinical evidence of hepatic decompensation. Prior to initiation of ribavirin, assess for underlying cardiac disease and refer to the ribavirin prescribing information [see Contraindications (4) and Warnings and Precautions (5.1 and 5.2)].
2.2 Recommended Dosage in Adults
TECHNIVIE is ombitasvir, paritaprevir and ritonavir fixed dose combination tablets.
The recommended dosage of TECHNIVIE is two tablets taken orally once daily (in the morning). Take TECHNIVIE with a meal without regard to fat or calorie content [see Clinical Pharmacology (12.3)].
TECHNIVIE is used in combination with ribavirin (RBV). When administered with TECHNIVIE, the recommended dosage of RBV is based on weight: 1000 mg per day for subjects less than 75 kg and 1200 mg per day for those weighing at least 75 kg, divided and administered twice-daily with food. For ribavirin dosage modifications, refer to the ribavirin prescribing information.
Table 1 shows the recommended TECHNIVIE treatment regimen and duration for HCV genotype 4 patients without cirrhosis or with compensated cirrhosis.
| Patient Population | Treatment | Duration |
| Genotype 4 without cirrhosis or with compensated cirrhosis (Child-Pugh A) | TECHNIVIE + ribavirin* | 12 weeks |
| *TECHNIVIE administered without RBV for 12 weeks may be considered for treatment-naïve patients without cirrhosis who cannot take or tolerate ribavirin [see Microbiology (12.4) and Clinical Studies (14)]. | ||
3 DOSAGE FORMS AND STRENGTHS
TECHNIVIE is a pink-colored, film-coated, oblong, biconvex-shaped tablet debossed “AV1” on one side. Each tablet contains 12.5 mg ombitasvir, 75 mg paritaprevir and 50 mg ritonavir.
4 CONTRAINDICATIONS
- The contraindications to ribavirin also apply to this combination regimen. Refer to the ribavirin prescribing information for a list of contraindications for ribavirin.
- TECHNIVIE is contraindicated:
- In patients with moderate to severe hepatic impairment (Child-Pugh B and C) due to risk of potential toxicity [see Warnings and Precautions (5.2), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- With drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events [see Drug Interactions (7) and Clinical Pharmacology (12.3)]:
- Alpha1-adrenoreceptor antagonist: alfuzosin HCL
- Anti-anginal: ranolazine
- Antiarrhythmic: dronedarone
- Anti-gout: colchicine in patients with renal and/or hepatic impairment
- Antipsychotic: lurasidone, pimozide
- Ergot derivatives: ergotamine, dihydroergotamine, methylergonovine
- Ethinyl estradiol-containing products such as combined oral contraceptives
- GI Motility Agent: cisapride
- HMG-CoA Reductase Inhibitors: atorvastatin, lovastatin, simvastatin
- Immunosuppressants: everolimus, sirolimus, tacrolimus
- Microsomal triglyceride transfer protein inhibitor: lomitapide
- Non-nucleoside reverse transcriptase inhibitor: efavirenz
- Phosphodiesterase-5(PDE5) inhibitor: sildenafil when dosed as Revatio for the treatment of pulmonary arterial hypertension (PAH)
- Sedatives/hypnotics: triazolam, orally administered midazolam
- With drugs that are moderate or strong inducers of CYP3A and may lead to reduced efficacy of TECHNIVIE [see Drug Interactions (7) and Clinical Pharmacology (12.3)]:
- Anticonvulsants: carbamazepine, phenytoin, phenobarbital
- Androgen receptor inhibitor: apalutamide
- Antimycobacterial: rifampin
- Herbal Product: St. John’s Wort (Hypericum perforatum)
- In patients with known hypersensitivity to ritonavir (e.g. toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome).
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
Hepatitis B virus (HBV) reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals, and who were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure and death. Cases have been reported in patients who are HBsAg positive and also in patients with serologic evidence of resolved HBV infection (i.e., HBsAg negative and anti-HBc positive). HBV reactivation has also been reported in patients receiving certain immunosuppressant or chemotherapeutic agents; the risk of HBV reactivation associated with treatment with HCV direct-acting antivirals may be increased in these patients.
HBV reactivation is characterized as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level. In patients with resolved HBV infection reappearance of HBsAg can occur. Reactivation of HBV replication may be accompanied by hepatitis, i.e., increases in aminotransferase levels and, in severe cases, increases in bilirubin levels, liver failure, and death can occur.
Test all patients for evidence of current or prior HBV infection by measuring HBsAg and anti-HBc before initiating HCV treatment with TECHNIVIE. In patients with serologic evidence of HBV infection, monitor for clinical and laboratory signs of hepatitis flare or HBV reactivation during HCV treatment with TECHNIVIE and during post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated.
5.2 Risk of Hepatic Decompensation and Hepatic Failure in Patients with Cirrhosis
Hepatic decompensation and hepatic failure, including liver transplantation or fatal outcomes, have been reported in clinical trials and postmarketing in patients treated with ombitasvir, paritaprevir, ritonavir with and without ribavirin. Most patients with these severe outcomes had evidence of cirrhosis prior to initiating therapy. Reported cases typically occurred within one to four weeks of initiating therapy and were characterized by the acute onset of rising direct serum bilirubin levels without ALT elevations in association with clinical signs and symptoms of hepatic decompensation.
TECHNIVIE is contraindicated in patients with moderate to severe hepatic impairment (Child-Pugh B and C) [see Contraindications (4), Adverse Reactions (6.2), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
For patients with compensated cirrhosis:
- Monitor for clinical signs and symptoms of hepatic decompensation (such as ascites, hepatic encephalopathy, variceal hemorrhage).
- Hepatic laboratory testing including direct bilirubin levels should be performed at baseline and during the first 4 weeks of starting treatment and as clinically indicated.
- Discontinue TECHNIVIE in patients who develop evidence of hepatic decompensation.
5.3 Increased Risk of ALT Elevations
During clinical trials with ombitasvir, paritaprevir and ritonavir with or without dasabuvir and with or without ribavirin, elevations of ALT to greater than 5 times the upper limit of normal (ULN) occurred in approximately 1% of subjects [see Adverse Reactions (6.1)]. ALT elevations were typically asymptomatic, occurred during the first 4 weeks of treatment, and declined within two to eight weeks of onset with continued dosing.
These ALT elevations were significantly more frequent in female subjects who were using ethinyl estradiol-containing medications such as combined oral contraceptives, contraceptive patches or contraceptive vaginal rings. Ethinyl estradiol-containing medications must be discontinued prior to starting therapy with TECHNIVIE [see Contraindications (4)]. Alternative methods of contraception (e.g., progestin only contraception or non-hormonal methods) are recommended during TECHNIVIE therapy. Ethinyl estradiol-containing medications can be restarted approximately 2 weeks following completion of treatment with TECHNIVIE.
Women using estrogens other than ethinyl estradiol, such as estradiol and conjugated estrogens used in hormone replacement therapy had a rate of ALT elevation similar to those not receiving any estrogens. Due to the limited number of subjects taking these other estrogens in clinical studies, caution is warranted for co-administration with TECHNIVIE [see Adverse Reactions (6.1)].
Hepatic laboratory testing should be performed during the first 4 weeks of starting treatment and as clinically indicated thereafter. If ALT is found to be elevated above baseline levels, it should be repeated and monitored closely:
- Patients should be instructed to consult their health care professional without delay if they have onset of fatigue, weakness, lack of appetite, nausea and vomiting, jaundice or discolored feces.
- Consider discontinuing TECHNIVIE if ALT levels remain persistently greater than 10 times the ULN.
- Discontinue TECHNIVIE if ALT elevation is accompanied by signs or symptoms of liver inflammation or increasing direct bilirubin, alkaline phosphatase, or INR.
5.4 Risks Associated With Ribavirin Combination Treatment
The warnings and precautions for ribavirin, in particular the pregnancy avoidance warning and use in patients with cardiac disease, apply to this combination regimen. Refer to the ribavirin prescribing information for a full list of the warnings and precautions for ribavirin [see Dosage and Administration (2.1)].
5.5 Risk of Adverse Reactions or Reduced Therapeutic Effect Due to Drug Interactions
The concomitant use of TECHNIVIE and certain other drugs may result in known or potentially significant drug interactions, some of which may lead to:
- Loss of therapeutic effect of TECHNIVIE and possible development of resistance
- Possible clinically significant adverse reactions from greater exposures of concomitant drugs or components of TECHNIVIE.
See Table 4 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during TECHNIVIE therapy; review concomitant medications during TECHNIVIE therapy; and monitor for the adverse reactions associated with the concomitant drugs [see Contraindications (4) and Drug Interactions (7)].
5.6 Risk of HIV-1 Protease Inhibitor Drug Resistance in HCV/HIV-1 Co-infected Patients
The ritonavir component of TECHNIVIE is also an HIV-1 protease inhibitor and can select for HIV-1 protease inhibitor resistance-associated substitutions. Any HCV/HIV-1 co-infected patients treated with TECHNIVIE should also be on a suppressive antiretroviral drug regimen to reduce the risk of HIV-1 protease inhibitor drug resistance.
6 ADVERSE REACTIONS
TECHNIVIE should be administered with ribavirin (RBV). Refer to the prescribing information for ribavirin for a list of ribavirin-associated adverse reactions.
The following adverse reaction is described below and elsewhere in the labeling:
- Risk of Hepatic Decompensation and Hepatic Failure in Patients with Cirrhosis [see Warnings and Precautions (5.2)]
- Increased Risk of ALT Elevations [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of ombitasvir, paritaprevir and ritonavir cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reaction in Subjects without Cirrhosis
The safety assessment of TECHNIVIE is based on data from two clinical studies in subjects with HCV genotype 4 infection. PEARL-I was a study including 135 subjects without cirrhosis, 91 who received ombitasvir 25 mg, paritaprevir 150 mg and ritonavir 100 mg (administered as one ombitasvir 25 mg tablet, three paritaprevir 50 mg tablets and one ritonavir 100 mg capsule) once daily with ribavirin for 12 weeks and 44 subjects who received ombitasvir 25 mg, paritaprevir 150 mg, and ritonavir 100 mg (administered as one ombitasvir 25 mg tablet, three paritaprevir 50 mg tablets and one ritonavir 100 mg capsule) once daily without ribavirin for 12 weeks.
Adverse reactions that occurred in subjects without cirrhosis treated with ombitasvir, paritaprevir and ritonavir with or without ribavirin for 12 weeks are listed in Table 2. The majority of adverse reactions in non-cirrhotic subjects were mild in severity, none were serious and none led to discontinuation of treatment.
| PEARL-I Without Cirrhosis |
||
| Adverse Reaction | Ombitasvir, paritaprevir, ritonavir + RBV N = 91 % | Ombitasvir, paritaprevir, ritonavir N = 44 % |
| Asthenia | 29 | 25 |
| Fatigue | 15 | 7 |
| Nausea | 14 | 9 |
| Insomnia | 13 | 5 |
| Pruritus1 | 7 | 5 |
| Skin reactions2,3 | 7 | 5 |
| 1Grouped term ‘pruritus’ includes the preferred terms pruritus and pruritus generalized. 2Grouped term ‘skin reactions’ includes the preferred terms rash, erythema, eczema, rash maculo-papular, rash macular, dermatitis, rash papular, skin exfoliation, rash pruritic, rash erythematous, rash generalized, dermatitis allergic, dermatitis contact, exfoliative rash, photosensitivity reaction, psoriasis, skin reaction, ulcer and urticaria. 3The majority of events were graded as mild in severity. |
||
Adverse Events in Subjects with Compensated Cirrhosis
AGATE-I was a study including 120 subjects with compensated cirrhosis who received TECHNIVIE once daily with ribavirin for a total of 12 weeks (n=60) or 16 weeks (n=60). Adverse events occurring up to and including 12 weeks of treatment (≤ 84 days) from both arms were included in the analysis of adverse events and are listed in Table 3.
Seven of 120 subjects (6%) experienced serious adverse events at or before 12 weeks of treatment. No adverse events led to the discontinuation of TECHNIVIE. Thirty-one subjects (26%) underwent ribavirin dose reductions; five discontinued ribavirin, three received transfusion and one received erythropoietin.
| AGATE-I Compensated Cirrhosis |
|
| Adverse Events | TECHNIVIE + RBV N=120 (%) |
| Fatigue | 25 |
| Asthenia | 25 |
| Headache | 23 |
| Musculoskeletal Pain/Changes1 | 17 |
| Pruritus | 16 |
| Insomnia/Sleep Disorder2 | 14 |
| Skin Reactions3 | 13 |
| Dyspnea4 | 11 |
| Mood Disorders5 | 11 |
| Nausea | 11 |
| Dizziness | 11 |
| Cardiac Events6 | 9 |
| Abdominal Pain7 | 9 |
| Cough | 7 |
| Clinical Liver or Bilirubin Related Events8 | 7 |
| Edema9 | 6 |
| Altered Mental Status10 | 6 |
| Decreased Appetite | 6 |
| Vomiting | 6 |
| 1Grouped term ‘musculoskeletal pain/changes’ includes the preferred terms arthralgia, arthritis, back pain, muscle injury, muscle spasms, muscular weakness, musculoskeletal chest pain, myalgia, neck pain, and pain in extremity. 2Grouped term ‘insomnia/sleep disorder’ includes preferred terms insomnia and sleep disorder. 3Grouped term ‘skin reactions’ includes preferred termsdermatitis bullous, dermatitis psoriasiform, dry skin, eczema asteatotic, erythema, rash, skin exfoliation, skin lesion and skin toxicity. 4Grouped term ‘dyspnea’ includes preferred terms dyspnea and dyspnea exertional. 5Grouped term ‘mood disorders’ includes preferred terms affective disorder, agitation, anxiety, depressed mood, depression, irritability, mania and suicide attempt. 6Grouped term ‘cardiac events’ includes preferred terms acute coronary syndrome, angina pectoris, atrial fibrillation, chest pain, hypertension, hypotension and palpitations. 7Grouped term ‘abdominal pain’ includes preferred terms abdominal discomfort, abdominal pain, abdominal pain lower and abdominal pain upper. 8Grouped term ‘clinical liver or bilirubin related events’ includes preferred terms ascites, hepatic encephalopathy, jaundice, ocular icterus, esophageal varices hemorrhage and portal vein thrombosis. 9Grouped term ‘edema’ includes preferred terms edema and edema peripheral. 10Grouped term ‘altered mental status’ includes preferred terms disturbance in attention, memory impairment, psychomotor retardation and somnolence. |
|
None of the 135 subjects without cirrhosis and two (2%) of the 120 subjects with compensated cirrhosis treated with TECHNIVIE experienced post-baseline serum ALT levels greater than 5 times the upper limit of normal (ULN) and ≥2 times baseline after starting treatment [see Warnings and Precautions (5.3)].
Serum Bilirubin Elevations in Patients without Cirrhosis
Post-baseline elevations in bilirubin at least 2 times ULN were observed in 5% (7/134) of subjects without cirrhosis, receiving TECHNIVIE, all of whom were also receiving RBV. These bilirubin increases were predominately indirect and related to the inhibition of the bilirubin transporters OATP1B1/1B3 by paritaprevir and possibly ribavirin-induced hemolysis. Bilirubin elevations occurred early after initiation of treatment, peaked by study Week 1, and generally resolved with ongoing therapy. Bilirubin elevations were generally not associated with serum ALT elevations.
Serum Bilirubin Elevations/Hepatic Decompensation in Patients with Compensated Cirrhosis
Among the 120 subjects with compensated cirrhosis, mean total bilirubin and mean indirect bilirubin levels increased to approximately 3 fold from baseline on treatment. Mean direct bilirubin levels increased to approximately 2 fold on treatment. Mean bilirubin elevations occurred early, peaked by Week 1, remained elevated on treatment and normalized by post treatment week 4. Bilirubin elevations were generally not associated with serum ALT elevations.
Over 40% (50/120) of subjects across both arms experienced elevated direct bilirubin levels (>ULN) at or before 12 weeks of treatment. Twelve percent (6/50) of these subjects experienced clinical bilirubin or liver related events including jaundice, ocular icterus and portal vein thrombosis.
One subject who did not have direct bilirubin elevations also experienced liver related adverse events of esophageal varices and ascites.
Anemia/Decreased Hemoglobin in Patients without Cirrhosis
The mean change from baseline in hemoglobin levels in subjects without cirrhosis treated with TECHNIVIE in combination with ribavirin was -2.1 g/dL and the mean change in subjects treated with TECHNIVIE alone was -0.4 g/dL. Decreases in hemoglobin levels occurred early in treatment (Week 1-2) with further reductions through Week 3. Hemoglobin values remained low during the remainder of treatment and returned towards baseline levels by post-treatment Week 4. One subject treated with TECHNIVIE with ribavirin had a single hemoglobin level decrease to less than 8 g/dL during treatment. No subject treated with TECHNIVIE alone had hemoglobin levels less than 8 g/dL. Four percent (4/91) of subjects without cirrhosis treated with TECHNIVIE with ribavirin underwent ribavirin dose reductions to manage anemia/decreased hemoglobin levels. No subject received erythropoietin.
Anemia/Decreased Hemoglobin in Patients with Compensated Cirrhosis
Across both treatment arms, 4/120 cirrhotic subjects (3%) had anemia (hemoglobin less than LLN) prior to initiation of treatment. However, 88/120 (73%) had anemia (hemoglobin less than LLN) and/or a hemoglobin decrease of ≥ 2g/dl at or before 12 weeks of treatment. One subject (1%) had a single hemoglobin value less than 8.0 g/dL on treatment at or before 12 weeks of treatment. Reductions in hemoglobin are most likely primarily related to ribavirin in this population.
Of 64 subjects with a history of cardiovascular disease or diabetes mellitus, 9 (14%) experienced cardiac adverse events at or before 12 weeks of treatment. These 9 subjects had a mean hemoglobin decrease of 3.9 g/dL (range 1.1 to 5.3 g/dL) from baseline and experienced cardiac events including acute coronary syndrome, angina pectoris, chest pain, atrial fibrillation, palpitations, hypotension and hypertension. Among 56 subjects without a prior history of cardiovascular disease or diabetes, 2 (4%) experienced a cardiac event (mild or moderate hypertension).
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of TECHNIVIE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Anaphylactic reactions and other hypersensitivity reactions (including angioedema).
Hepatobiliary Disorders: Hepatic decompensation, hepatic failure [see Warnings and Precautions (5.3)].
Skin and Subcutaneous Tissue Disorders: Erythema multiforme (EM).
7 DRUG INTERACTIONS
7.1 Potential for TECHNIVIE to Affect Other Drugs
Paritaprevir is an inhibitor of OATP1B1 and OATP1B3 and paritaprevir and ritonavir are inhibitors of BCRP and P-gp. Ritonavir is an inhibitor of CYP3A4. Co-administration of TECHNIVIE with drugs that are substrates of CYP3A, P-gp, BCRP, OATP1B1 or OATP1B3 may result in increased plasma concentrations of such drugs [see also Contraindications (4), Warnings and Precautions (5.5), and Clinical Pharmacology (12.3)].
7.2 Potential for Other Drugs to Affect One or More Components of TECHNIVIE
Paritaprevir and ritonavir are primarily metabolized by CYP3A enzymes. Co-administration of TECHNIVIE with strong inhibitors of CYP3A may increase paritaprevir and ritonavir concentrations. Ombitasvir is primarily metabolized via amide hydrolysis while CYP enzymes play a minor role in its metabolism. Ombitasvir, paritaprevir and ritonavir are substrates of P-gp. Paritaprevir is a substrate of BCRP, OATP1B1 and OATP1B3. Inhibition of P-gp, BCRP, OATP1B1 or OATP1B3 may increase the plasma concentrations of the various components of TECHNIVIE.
7.3 Established and Other Potential Drug Interactions
Clearance of HCV infection with direct-acting antivirals may lead to changes in hepatic function, which may impact the safe and effective use of concomitant medications. For example, altered blood glucose control resulting in serious symptomatic hypoglycemia has been reported in diabetic patients in postmarketing case reports and published epidemiological studies. Management of hypoglycemia in these cases required either discontinuation or dose modification of concomitant medications used for diabetes treatment.
Frequent monitoring of relevant laboratory parameters (e.g. International Normalized Ratio [INR] in patients taking warfarin, blood glucose levels in diabetic patients) or drug concentrations of concomitant medications such as CYP P450 substrates with a narrow therapeutic index (e.g. certain immunosuppressants) is recommended to ensure safe and effective use. Dose adjustments of concomitant medications may be necessary.
If dosage adjustments of concomitant medications are made due to treatment with TECHNIVIE, dosages should be re-adjusted after administration of TECHNIVIE is completed. Dosage adjustment is not required for TECHNIVIE.
Table 4 provides the effect of co-administration of TECHNIVIE on concentrations of concomitant drugs and the effect of concomitant drugs on the various components of TECHNIVIE. See Contraindications (4) for drugs that are contraindicated with TECHNIVIE. Refer to the ritonavir prescribing information for other potentially significant drug interactions with ritonavir.
| Concomitant Drug Class: Drug Name | Effect on Concentration | Clinical Comments |
| ALPHA1-ADRENORECEPTOR ANTAGONIST | ||
| alfuzosin HCl* | ↑ alfuzosin HCl | Contraindicated due to potential for hypotension [see Contraindications (4)]. |
| ANDROGEN RECEPTOR INHIBITOR | ||
| apalutamide* | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| ANGIOTENSIN RECEPTOR BLOCKERS e.g. | ||
| valsartan*, losartan*, candesartan* | ↑ angiotensin receptor blockers | Decrease the dose of the angiotensin receptor blockers and monitor patients for signs and symptoms of hypotension and/or worsening renal function. If such events occur, consider further dose reduction of the angiotensin receptor blocker or switching to an alternative to the angiotensin receptor blocker. |
| ANTI-ANGINAL | ||
| ranolazine* | ↑ ranolazine | Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. |
| ANTIARRHYTHMICS | ||
| dronedarone* | ↑ dronedarone | Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
| digoxin | ↑ digoxin | For contraindicated antiarrhythmics [see Contraindications (4)]. Decrease digoxin dose by 30-50%. Appropriate monitoring of serum digoxin levels is recommended. |
| amiodarone*, bepridil*, disopyramide*, flecainide*, lidocaine (systemic)*, mexiletine*, propafenone*, quinidine* | ↑ antiarrhythmics | Therapeutic monitoring (if available) is recommended for antiarrhythmics when co-administered with TECHNIVIE. |
| ANTICANCER AGENTS/KINASE INHIBITORS | ||
| encorafenib* fostamatinib* ibrutinib* ivosidenib* | ↑ anticancer agents/kinase inhibitors | Co-administration of TECHNIVIE with these anticancer agents/kinase inhibitors may result in increased risk for adverse events. Refer to the prescribing information of these agents for details on co-administration with strong CYP3A inhibitors. |
| ANTICONVULSANTS | ||
| carbamazepine*, phenytoin*, phenobarbital* | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| ANTIDIABETIC DRUGS | ||
| metformin | ↔ metformin | Monitor for signs of onset of lactic acidosis such as respiratory distress, somnolence, and non-specific abdominal distress or worsening renal function. Concomitant metformin use in patients with renal insufficiency or hepatic impairment is not recommended. Refer to the prescribing information of metformin for further guidance. |
| ANTI-GOUT | ||
| colchicine* | ↑ colchicine | Contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment [see Contraindications (4)]. |
| ANTIFUNGALS | ||
| ketoconazole | ↑ ketoconazole | When TECHNIVIE is co-administered with ketoconazole, the maximum daily dose of ketoconazole should be limited to 200 mg per day. |
| voriconazole* | ↓ voriconazole | Co-administration of TECHNIVIE with voriconazole is not recommended unless an assessment of the benefit-to-risk ratio justifies the use of voriconazole. |
| ANTIMYCOBACTERIAL | ||
| rifampin* | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| ANTIPSYCHOTICS | ||
| lurasidone* | ↑ lurasidone | Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. |
| pimozide* | ↑ pimozide | Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
| quetiapine* | ↑ quetiapine | For contraindicated antipsychotics [see Contraindications (4)].
|
| CALCIUM CHANNEL BLOCKERS | ||
| amlodipine, nifedipine*, diltiazem*, verapamil* | ↑ calcium channel blockers | Decrease the dose of the calcium channel blocker. The dose of amlodipine should be decreased by at least 50%. Clinical monitoring of patients is recommended for edema and/or signs and symptoms of hypotension. If such events occur, consider further dose reduction of the calcium channel blocker or switching to an alternative to the calcium channel blocker. |
| CORTICOSTEROIDS (INHALED/NASAL) | ||
| fluticasone* | ↑ fluticasone | Concomitant use of TECHNIVIE with inhaled or nasal fluticasone may reduce serum cortisol concentrations. Alternative corticosteroids should be considered, particularly for long term use. |
| DIURETICS | ||
| furosemide | ↑ furosemide (Cmax) | Clinical monitoring of patients is recommended and therapy should be individualized based on patient’s response. |
| ERGOT DERIVATIVES | ||
| ergotamine*, dihydroergotamine*, methylergonovine* | ↑ ergot derivatives | Contraindicated due to potential for acute ergot toxicity characterized by vasospasm. Tissue ischemia has been associated with co-administration of ritonavir and ergonovine, ergotamine, dihydroergotamine, or methylergonovine [see Contraindications (4)]. |
| ETHINYL ESTRADIOL-CONTAINING PRODUCTS | ||
| ethinyl estradiol- containing medications such as combined oral contraceptives | ↔ ethinyl estradiol | Contraindicated due to potential for ALT elevations [see Contraindications (4) and Warnings and Precautions (5.3)]. |
| GI MOTILITY AGENT | ||
| cisapride* | ↑ cisapride | Contraindicated due to potential for serious and/or life threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
| GnRH RECEPTOR ANTAGONISTS | ||
| elagolix* | ↑ elagolix | Co-administration of TECHNIVIE with elagolix 200 mg twice daily for more than 1 month is not recommended. |
| HERBAL PRODUCT | ||
| St. John’s Wort* (Hypericum perforatum) | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| HIV-ANTIVIRAL AGENTS | ||
| efavirenz* | ↑ efavirenz ↓ paritaprevir ↓ ritonavir | Contraindicated as co-administration of efavirenz based regimens with paritaprevir and ritonavir was poorly tolerated and resulted in liver enzyme elevations [see Contraindications (4)]. |
| atazanavir or atazanavir/ritonavir | ↑ paritaprevir | Co-administration of TECHNIVIE with atazanavir or atazanavir/ritonavir is not recommended. |
| darunavir/ritonavir | ↓ darunavir (Ctrough) | Treatment naïve patients or treatment experienced patients with no darunavir-associated mutations: Darunavir 800 mg once daily (without ritonavir) can be co-administered with TECHNIVIE. |
| lopinavir/ritonavir | ↑ paritaprevir | Co-administration of TECHNIVIE with lopinavir/ritonavir is not recommended. |
| rilpivirine | ↑ rilpivirine | For contraindicated non-nucleoside reverse transcriptase inhibitors [see Contraindications (4)]. Co-administration of TECHNIVIE with rilpivirine once daily is not recommended due to potential for QT interval prolongation with higher concentrations of rilpivirine. |
| HMG CoA REDUCTASE INHIBITORS | ||
| atorvastatin lovastatin, simvastatin | ↑ atorvastatin ↑ lovastatin, ↑ simvastatin | Contraindicated due to potential for myopathy including rhabdomyolysis [see Contraindications (4)]. |
| pravastatin | ↑ pravastatin | For contraindicated HMG CoA Reductase Inhibitors [see Contraindications (4)]. When TECHNIVIE is co-administered with pravastatin, the dose of pravastatin should not exceed 40 mg per day. |
| IMMUNOSUPPRESSANTS | ||
| everolimus sirolimus tacrolimus | ↑ everolimus ↑ sirolimus ↑ tacrolimus | Contraindicated due to potential for serious and/or life threatening immunosuppressant-associated adverse events [see Contraindications (4)]. |
| cyclosporine | ↑ cyclosporine | For contraindicated immunosuppressants [see Contraindications (4)]. When initiating therapy with TECHNIVIE, reduce cyclosporine dose to 1/5th of the patient’s current cyclosporine dose. Measure cyclosporine blood concentrations to determine subsequent dose modifications. Upon completion of TECHNIVIE therapy, the appropriate time to resume pre-TECHNIVIE dose of cyclosporine should be guided by assessment of cyclosporine blood concentrations. Frequent assessment of renal function and cyclosporine-related side effects is recommended. |
| LONG ACTING BETA-ADRENOCEPTOR AGONIST | ||
| salmeterol* | ↑ salmeterol | Concurrent administration of TECHNIVIE and salmeterol is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. |
| MUSCLE RELAXANTS | ||
| carisoprodol | ↓ carisoprodol ↔ mepobramate (metabolite of carisoprodol) | Increase dose if clinically indicated. |
| cyclobenzaprine | ↓cyclobenzaprine ↓norcyclobenzaprine (metabolite of cyclobenzaprine) | Increase dose if clinically indicated. |
| MICROSOMAL TRIGLYCERIDE TRANSFER PROTEIN INHIBITOR | ||
| lomitapide* | ↑ lomitapide | Contraindicated due to potential for serious adverse events including hepatotoxicity [see Contraindications (4)]. |
| NARCOTIC ANALGESICS | ||
| buprenorphine/naloxone | ↑ buprenorphine ↑ norbuprenorphine (metabolite of buprenorphine) | Patients should be closely monitored for sedation and cognitive effects. |
| hydrocodone/ acetaminophen fentanyl | ↑ hydrocodone ↔ acetaminophen ↑ fentanyl | Reduce the dose of hydrocodone by 50% and monitor patients for respiratory depression and sedation at frequent intervals. Upon completion of TECHNIVIE therapy, adjust the hydrocodone dose and monitor for signs of opioid withdrawal. Careful monitoring of therapeutic effects and adverse effects of fentanyl (including potentially fatal respiratory depression) is recommended when fentanyl is co-administered with TECHNIVIE. |
| PROTON PUMP INHIBITORS | ||
| omeprazole | ↓ omeprazole | Monitor patients for decreased efficacy of omeprazole. Consider increasing the omeprazole dose in patients whose symptoms are not well controlled; avoid use of more than 40 mg per day of omeprazole. |
| PHOSPHODIESTERASE-5 (PDE5) INHIBITOR | ||
| Sildenafil* when dosed as Revatio for the treatment of pulmonary arterial hypertension (PAH) | ↑ sildenafil | Contraindicated due to potential for sildenafil-associated adverse events such as visual disturbances, hypotension, priapism, and syncope [see Contraindications (4)]. |
| SEDATIVES/HYPNOTICS | ||
| triazolam* orally administered midazolam* | ↑ triazolam ↑ midazolam | Contraindicated due to potential for serious and/or life threatening events such as prolonged or increased sedation or respiratory depression [see Contraindications (4)]. |
| alprazolam | ↑ alprazolam | For contraindicated Sedatives/Hypnotics [see Contraindications (4)]. Clinical monitoring of patients is recommended. A decrease in alprazolam dose can be considered based on clinical response. |
| diazepam | ↓ diazepam ↓ nordiazepam (metabolite of diazepam) | Increase dose if clinically indicated. |
| *Not studied. See Clinical Pharmacology, Tables 7 and 8. The direction of the arrow indicates the direction of the change in exposures (Cmax and AUC) (↑ = increase of more than 20%, ↓ = decrease of more than 20%). |
||
7.4 Drugs without Clinically Significant Interactions with TECHNIVIE
No dosage adjustments are recommended when TECHNIVIE is co-administered with the following medications: abacavir, dolutegravir, duloxetine, emtricitabine/tenofovir disoproxil fumarate, escitalopram, gemfibrozil, lamivudine, methadone, progestin only contraceptives, raltegravir, sofosbuvir, sulfamethoxazole, trimethoprim, rosuvastatin, and zolpidem.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
If TECHNIVIE is administered with ribavirin, the combination regimen is contraindicated in pregnant women and in men whose female partners are pregnant. Refer to the ribavirin prescribing information for more information on use in pregnancy.
No adequate human data are available to establish whether or not TECHNIVIE poses a risk to pregnancy outcomes. In animal reproduction studies, no adverse developmental effects were observed when the components of TECHNIVIE were administered separately during organogenesis and lactation. During organogenesis, the exposures were up to 29 and 4 times (mice and rabbits, respectively; ombitasvir), 143 and 12 times (mice and rats, respectively; paritaprevir, ritonavir) exposures at the recommended clinical dose of TECHNIVIE. In rodent pre/postnatal developmental studies, maternal systemic exposures (AUC) to ombitasvir and paritaprevir were approximately 26 and 24 times, respectively, the exposure in humans at the recommended clinical dose [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Ombitasvir was administered orally to pregnant mice (0, 15, 50, or 150 mg/kg/day) and rabbits (0, 10 or 60 mg/kg/day) during the period of organogenesis (on gestation days (GD) 6 to 15, and GD 7 to 19, respectively). There were no ombitasvir-related maternal or embryofetal effects (malformations or fetal toxicity) at any dose level in either species. The systemic exposures at the highest doses were 29-times higher (mice) and 4-times higher (rabbits) than the exposures in humans at the recommended clinical dose.
In a pre- and postnatal developmental study in mice, ombitasvir was administered orally at 0, 10, 40, or 200 mg/kg/day from GD 6 to lactation day 20. There were no ombitasvir-related effects at maternal exposures 26-times higher than exposures in humans at the recommended clinical dose.
The major human metabolites of ombitasvir, M29 and M36, were tested in pregnant mice during the period of organogenesis from GD 6 to 15. M29 was administered orally at doses of 0, 1, 2.5 or 4.5 mg/kg/day. M36 was dosed orally at doses 1.5, 3, or 6 mg/kg/day. In both cases, there were no treatment related maternal effects or embryofetal effects (malformations or fetal toxicity) at any dose level. The highest doses produced exposures approximately 26-times higher than the exposures in humans at the recommended clinical dose.
Paritaprevir/ritonavir was administered orally to pregnant rats (0/0, 30/15, 100/15, 450/45 mg/kg/day) and mice (0/0, 30/30, 100/30, or 300/30 mg/kg/day) during the period of organogenesis (on GD 6 to 17, and GD 6 to 15, respectively). There were no test article-related maternal or embryofetal effects (malformations or fetal toxicity) at any dose level in either species. The highest systemic exposure of paritaprevir was 12-times higher (rats) and 143-times higher (mice) than the exposures in humans at the recommended clinical dose.
In a pre- and postnatal developmental study in rats, paritaprevir/ritonavir were administered orally at 0/0, 6/30, 30/30, or 300/30 mg/kg/day from GD 7 to lactation day 20. There were no treatment related effects at maternal exposures 24-times higher than exposures in humans at the recommended clinical dose.
8.2 Lactation
It is not known whether TECHNIVIE and its metabolites are present in human breast milk, affect human milk production or have effects on the breastfed infant. Unchanged ombitasvir, paritaprevir and its hydrolysis product M13 were the predominant components observed in the milk of lactating rats, without effect on nursing pups [see Data].
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for TECHNIVIE and any potential adverse effects on the breastfed child from TECHNIVIE or from the underlying maternal condition.
If TECHNIVIE is administered with ribavirin, the nursing mother’s information for ribavirin also applies to this combination regimen. Refer to the ribavirin prescribing information for more information on use during lactation.
No effects of ombitasvir on growth and postnatal development were observed in nursing pups at the highest dose tested, 200 mg/kg/day [see Data in (8.1)]. Maternal systemic exposure (AUC) to ombitasvir was approximately 26 times the exposure in humans at the recommended clinical dose. Although not measured directly, ombitasvir was likely present in the milk of lactating mice in this study, since systemic exposure was observed in nursing pups on post-natal day 21 (approximately 3-16 % of the maternal exposure).
When administered to lactating rats 10 to 11 days after parturition at a dose of 5 mg/kg, the 24 hr AUC in milk was 4 times higher than in plasma and the majority of the radioactivity in the milk was unchanged parent drug (91%).
No effects of paritaprevir/ritonavir on growth and postnatal development were observed in nursing pups at the highest dose tested 300/30 mg/kg/day paritaprevir/ritonavir [see Data in (8.1)]. Maternal systemic exposure (AUC) to paritaprevir was approximately 24 times the exposure in humans at the recommended clinical dose. Although not measured directly, paritaprevir was likely present in the milk of lactating rats at the high dose in this study, since systemic exposure was observed in nursing pups on post-natal day 15 (approximately 0.3 % of the maternal exposure).
When administered to lactating rats 10 to 11 days after parturition at a dose of 30/15 mg/kg paritaprevir/ritonavir, the 24 hr AUC in milk was half that in plasma and the majority of the radioactivity in the milk was the hydrolysis product M13 (84%) followed by unchanged parent drug (16%).
8.3 Females and Males of Reproductive Potential
If TECHNIVIE is administered with ribavirin, the information for ribavirin with regard to pregnancy testing, contraception, and infertility also applies to this combination regimen. Refer to ribavirin prescribing information for additional information.
8.4 Pediatric Use
Safety and effectiveness of TECHNIVIE in pediatric patients less than 18 years of age have not been established.
8.5 Geriatric Use
No dosage adjustment of TECHNIVIE is warranted in geriatric patients. Clinical studies PEARL-I and AGATE-1 did not include sufficient numbers of patients older than 65 years of age to assess safety or efficacy, or to determine if they responded differently than younger patients.
8.6 Hepatic Impairment
No dosage adjustment of TECHNIVIE is required in patients with mild hepatic impairment (Child-Pugh A). TECHNIVIE is contraindicated in patients with moderate to severe hepatic impairment (Child-Pugh B and C) [see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment of TECHNIVIE is required in patients with mild, moderate or severe renal impairment. TECHNIVIE has not been studied in patients on dialysis. For patients that require ribavirin, refer to the ribavirin prescribing information for information regarding use in patients with renal impairment [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In case of overdose, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions and appropriate symptomatic treatment be instituted immediately.
11 DESCRIPTION
TECHNIVIE is a fixed-dose combination tablet containing ombitasvir, paritaprevir, and ritonavir for oral administration.
Ombitasvir, paritaprevir, ritonavir fixed dose combination tablet includes a hepatitis C virus NS5A inhibitor (ombitasvir), a hepatitis C virus NS3/4A protease inhibitor (paritaprevir), and a CYP3A inhibitor (ritonavir) that inhibits CYP3A mediated metabolism of paritaprevir, thereby providing increased plasma concentration of paritaprevir.
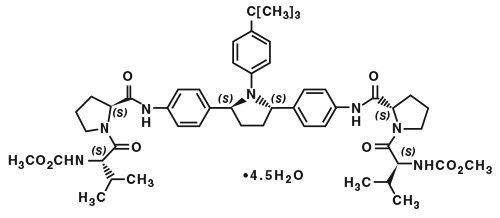
The chemical name of ombitasvir is Dimethyl ([(2S,5S)-1-(4-tert-butylphenyl) pyrrolidine-2,5-diyl]bis{benzene-4,1-diylcarbamoyl(2S)pyrrolidine-2,1-diyl[(2S)-3-methyl-1-oxobutane-1,2-diyl]})biscarbamate hydrate. The molecular formula is C50H67N7O8•4.5H2O (hydrate) and the molecular weight for the drug substance is 975.20 (hydrate). The drug substance is white to light yellow to light pink powder, and is practically insoluble in aqueous buffers but is soluble in ethanol. Ombitasvir has the following molecular structure:
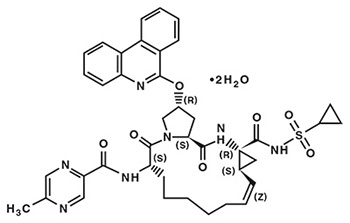
The chemical name of paritaprevir is (2R,6S,12Z,13aS,14aR,16aS)-N-(cyclopropylsulfonyl)-6-{[(5-methylpyrazin-2-yl)carbonyl]amino}-5,16-dioxo-2-(phenanthridin-6-yloxy)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydrocyclopropa[e]pyrrolo[1,2-a][1,4] diazacyclopentadecine-14a(5H)-carboxamide dihydrate. The molecular formula is C40H43N7O7S•2H2O (dihydrate) and the molecular weight for the drug substance is 801.91 (dihydrate). The drug substance is white to off-white powder with very low water solubility. Paritaprevir has the following molecular structure:
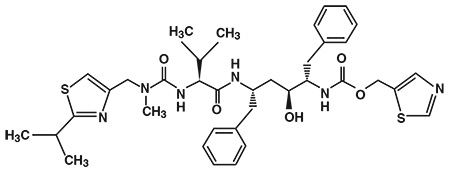
The chemical name of ritonavir is [5S-(5R*,8R*,10R*,11R*)]10-Hydroxy-2-methyl-5-(1-methyethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12-tetraazatridecan-13-oic acid,5-thiazolylmethyl ester. The molecular formula is C37H48N6O5S2 and the molecular weight for the drug substance is 720.95. The drug substance is white to off white to light tan powder practically insoluble in water and freely soluble in methanol and ethanol. Ritonavir has the following molecular structure:
Ombitasvir, Paritaprevir, Ritonavir Fixed-Dose Combination Tablets
Ombitasvir, paritaprevir and ritonavir film-coated tablets are co-formulated immediate release tablets. The tablet contains copovidone, K value 28, vitamin E polyethylene glycol succinate, propylene glycol monolaurate Type I, sorbitan monolaurate, colloidal silicon dioxide/colloidal anhydrous silica, sodium stearyl fumarate, polyvinyl alcohol, polyethylene glycol 3350/macrogol 3350, talc, titanium dioxide, and iron oxide red. The strength for the tablet is 12.5 mg ombitasvir, 75 mg paritaprevir, 50 mg ritonavir.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
TECHNIVIE combines two direct-acting hepatitis C virus antiviral agents with distinct mechanisms of action [see Microbiology (12.4)].
Ritonavir is not active against HCV. Ritonavir is a potent CYP3A inhibitor that increases peak and trough plasma drug concentrations of paritaprevir and overall drug exposure (i.e., area under the curve).
12.2 Pharmacodynamics
The effect of a combination of ombitasvir, paritaprevir and ritonavir plus dasabuvir on QTc interval was evaluated in a randomized, double blind, placebo and active-controlled (moxifloxacin 400 mg) 4-way crossover thorough QT study in 60 healthy subjects. At concentrations approximately 6 and 1.8 times the therapeutic concentrations of paritaprevir and ombitasvir, the combination did not prolong QTc to any clinically relevant extent.
12.3 Pharmacokinetics
The pharmacokinetic properties of the components of TECHNIVIE are provided in Table 5. Based on the population pharmacokinetic analysis, the median steady-state pharmacokinetic parameters of ombitasvir, paritaprevir, and ritonavir in HCV-infected subjects are provided in Table 6.
| Ombitasvir | Paritaprevir | Ritonavir | |
| Absorption | |||
| Tmax (hr) | ~ 5 | ~ 4-5 | ~ 4-5 |
| Absolute bioavailability (%) | 48 | 53 | NA |
| Effect of moderate fat meal (relative to fasting)a | 1.82 (1.61-2.05) | 3.11 (2.16-4.46) | 1.49 (1.23-1.79) |
| Effect of high fat meal (relative to fasting)a | 1.76 (1.56-1.99) | 2.80 (1.95-4.02) | 1.44 (1.19-1.73) |
| Accumulationb | 0.90- to 1.03-fold | 1.5- to 2-fold | |
| Distribution | |||
| % Bound to human plasma proteins | 99.9 | 97-98.6 | >99 |
| Blood-to-plasma ratio | 0.49 | 0.7 | 0.6 |
| Volume of distribution at steady state (Vss) (L) | 173 | 103 | 21.5c |
| Metabolism | |||
| Metabolism | amide hydrolysis followed by oxidative metabolism | CYP3A4 (major), CYP3A5 | CYP3A (major), CYP2D6 |
| Eliminationd | |||
| Major route of elimination | biliary excretion | metabolism | metabolism |
| t1/2 (hr)e | 21-25 | 5.5 | 4 |
| % of dose excreted in fecesf | 90.2 | 88 | 86.4 |
| % of dose excreted unchanged in fecesf | 87.8 | 1.1 | 33.8 |
| % of dose excreted in urinef | 1.91 | 8.8 | 11.3 |
| % of dose excreted unchanged in urinef | 0.03 | 0.05 | 3.5 |
| NA - data not available a. Values refer to mean non-fasting/fasting ratios (90% Confidence Interval) in systemic exposure (AUC). Moderate fat meal ~600 Kcal, 20-30% calories from fat. High fat meal ~900 Kcal, 60% calories from fat. b. Steady state exposures are achieved after approximately 12 days of dosing. c. It is apparent volume of distribution (V/F) for ritonavir. d. Ombitasvir, paritaprevir, and ritonavir do not inhibit organic anion transporter (OAT1) in vivo and based on in vitro data, are not expected to inhibit organic cation transporter (OCT2), organic anion transporter (OAT3), or multidrug and toxin extrusion proteins (MATE1 and MATE2K) at clinically relevant concentrations. e. t1/2 values refer to the mean elimination half-life. f. Dosing in mass balance studies: single dose administration of [14C]ombitasvir; single dose administration of [14C]paritaprevir co-dosed with 100 mg ritonavir. |
|||
| Pharmacokinetic Parametera | Ombitasvir | Paritaprevir | Ritonavir |
| Cmax (ng/mL) | 82 | 194 | 543 |
| AUC0-24 (ng*h/mL) | 1239 | 2276 | 6072 |
| a. Median values reported based on the population PK analysis. | |||
The single dose pharmacokinetics of ombitasvir, paritaprevir, ritonavir and another antiviral drug were evaluated in non-HCV infected subjects with mild hepatic impairment (Child-Pugh A; score of 5-6), moderate hepatic impairment (Child-Pugh B, score of 7-9) and severe hepatic impairment (Child-Pugh C, score of 10-15).
Relative to subjects with normal hepatic function, ombitasvir, paritaprevir and ritonavir mean AUC values decreased by 8%, 29% and 34%, respectively, in subjects with mild hepatic impairment.
Relative to subjects with normal hepatic function, ombitasvir and ritonavir mean AUC values decreased by 30% and 30%, respectively and paritaprevir mean AUC values increased by 62% in subjects with moderate hepatic impairment.
Relative to subjects with normal hepatic function, paritaprevir and ritonavir mean AUC values increased by 945% and 13% respectively and ombitasvir mean AUC values decreased by 54% in subjects with severe hepatic impairment [see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.2) and Use in Specific Populations (8.6)].
The single dose pharmacokinetics of ombitasvir, paritaprevir and ritonavir were evaluated in non-HCV infected subjects with mild (CLcr: 60 to 89 mL/min), moderate (CLcr: 30 to 59 mL/min), and severe (CLcr: 15 to 29 mL/min) renal impairment.
Overall, changes in exposure of ombitasvir, paritaprevir, and ritonavir in non-HCV infected subjects with mild-, moderate- and severe renal impairment are not expected to be clinically relevant. Pharmacokinetic data are not available on the use of TECHNIVIE in non-HCV infected subjects with End Stage Renal Disease (ESRD).
Relative to subjects with normal renal function, ritonavir AUC values increased by 40%, while ombitasvir and paritaprevir AUC values were unchanged in subjects with mild renal impairment.
Relative to subjects with normal renal function, ritonavir AUC values increased by 76%, while ombitasvir and paritaprevir AUC values were unchanged in subjects with moderate renal impairment.
Relative to subjects with normal renal function, paritaprevir and ritonavir AUC values increased by 25% and 108%, respectively, while ombitasvir AUC values were unchanged in subjects with severe renal impairment.
The pharmacokinetics of TECHNIVIE in pediatric patients less than 18 years of age has not been established [see Use in Specific Populations (8.4)].
No dosage adjustment is recommended based on sex or body weight.
No dosage adjustment is recommended based on race or ethnicity.
No dosage adjustment is recommended in geriatric patients [see Use in Specific Populations (8.5)].
See also Contraindications (4), Warnings and Precautions (5.5), Drug Interactions (7)
The effects of drugs discussed in Table 4 on the exposures of the individual components of TECHNIVIE are shown in Table 7. For information regarding clinical recommendations, see Drug Interactions (7).
Table 8 summarizes the effects of TECHNIVIE on the pharmacokinetics of co-administered drugs which showed clinically relevant changes. For information regarding clinical recommendations, see Drug Interactions (7).
12.4 Microbiology
TECHNIVIE combines two direct-acting antiviral agents with distinct mechanisms of action and non-overlapping resistance profiles to target HCV at multiple steps in the viral lifecycle.
Ombitasvir is an inhibitor of HCV NS5A, which is essential for viral RNA replication and virion assembly. The mechanism of action of ombitasvir has been characterized based on cell culture antiviral activity and drug resistance mapping studies.
Paritaprevir is an inhibitor of HCV NS3/4A protease which is necessary for the proteolytic cleavage of the HCV encoded polyprotein (into mature forms of the NS3, NS4A, NS4B, NS5A, and NS5B proteins) and is essential for viral replication. In a biochemical assay, paritaprevir inhibited the proteolytic activity of a recombinant HCV genotype 4a NS3/4A protease enzyme with an IC50 value of 0.16 nM.
The EC50 values of ombitasvir against HCV replicons containing NS5A from a single isolate each of genotype 4a and genotype 4d were 1.7 pM and 0.38 pM, respectively. Ombitasvir had median EC50 values of 0.21 pM (range 0.10 pM to 0.36 pM; n=9) and 35.6 pM (range 0.14 pM to 71 pM; n=2) against transient HCV replicons containing NS5A genes from a panel of genotype 4a and genotype 4b clinical isolates, respectively, from treatment-naïve subjects.
The EC50 values of paritaprevir against HCV replicons containing NS3 from a single isolate each of genotype 4a and genotype 4d were 0.09 nM and 0.015 nM, respectively.
In HCV replicon cell culture assays, ritonavir did not exhibit a direct antiviral effect and the presence of ritonavir did not affect the antiviral activity of paritaprevir.
Exposure of HCV genotype 4a replicons to ombitasvir or paritaprevir resulted in the emergence of drug resistant replicons carrying amino acid substitutions in NS5A or NS3, respectively. Amino acid substitutions in NS5A or NS3 selected in cell culture or identified in clinical study PEARL-I were phenotypically characterized in genotype 4 replicons.
For ombitasvir, in an HCV genotype 4a replicon, NS5A substitution L28V reduced ombitasvir antiviral activity by 21-fold. In an HCV genotype 4d replicon, substitutions L28V alone and L28V in combination with T58S reduced ombitasvir antiviral activity by 310- and 760-fold, respectively. Ombitasvir activity against an HCV genotype 4d replicon was not reduced by a T58P polymorphism, which represents the consensus sequence observed at this position for HCV genotype 4a and 4d subjects in PEARL-I. The presence of NS5A L30S and Y93H polymorphisms in a genotype 4b clinical isolate was associated with approximately 200-fold reduced activity of ombitasvir.
For paritaprevir, in an HCV genotype 4a replicon, NS3 substitutions R155C, A156T/V, and D168H/V reduced paritaprevir antiviral activity by 40- to 323-fold. In an HCV genotype 4d replicon, NS3 substitutions Y56H and D168V reduced paritaprevir antiviral activity by 8- and 313-fold, respectively, while a combination of Y56H and D168V reduced the activity of paritaprevir by 12,533-fold.
In the clinical study PEARL-I, three subjects experienced virologic failure (2 post-treatment relapse, 1 on-treatment failure). All 3 virologic failures were observed in a regimen containing paritaprevir/ritonavir and ombitasvir without ribavirin. Treatment-emergent, resistance-associated substitutions were detected at the time of failure in all 3 subjects and included D168V (with or without Y56H) in NS3, and L28S and L28V (with or without M31I or T58S) in NS5A.
In the clinical study AGATE-I, one subject experienced on-treatment virologic failure and a second subject experienced virologic relapse at the Post-Treatment Week 24 timepoint. Treatment-emergent resistance-associated substitutions detected in these subjects included NS3 substitutions A156K or D168V, and NS5A substitutions K24Q, L28I/M, M31I or Y93H.
Persistence of Resistance-Associated Substitutions
The persistence of ombitasvir and paritaprevir treatment-emergent amino acid substitutions in NS5A and NS3, respectively, was assessed in subjects in clinical study PEARL-I whose virus had at least 1 treatment-emergent resistance-associated substitution in the drug target, and with available data through at least 24 weeks post-treatment. Population and clonal nucleotide sequence analyses (assay sensitivity approximately 5-10%) were conducted to detect the persistence of viral populations with treatment-emergent substitutions. Available data were from 3 subjects with genotype 4d infection.
For ombitasvir, viral populations with 1 or more resistance-associated treatment-emergent substitutions in NS5A persisted at detectable levels in 3/3 (100%) subjects at Post-Treatment Week 24 and in 2/3 (67%) subjects through Post-Treatment Week 48. For paritaprevir, viral populations with 1 or more resistance-associated treatment-emergent substitutions in NS3 were detected through Post-Treatment Week 24 in 2/3 (67%) subjects, and in 0/3 (0%) subjects through Post-Treatment Week 48.
Effect of Baseline HCV Polymorphisms on Treatment Response
Phylogenetic analysis of HCV nucleotide sequences from subjects in the clinical study PEARL-I, identified 7 HCV genotype 4 subtypes (4a, 4b, 4c, 4d, 4f, 4g/4k, 4o). Most subjects were infected with either subtype 4a (38%) or 4d (52%); 1 to 7 subjects were infected with each of the other genotype 4 subtypes. Among subjects enrolled at U.S. study sites, 16/18 (89%) were infected with HCV subtype 4a; one subject each was infected with subtype 4c or 4d. Three subjects who experienced virologic failure with the regimen containing paritaprevir/ritonavir and ombitasvir without ribavirin were infected with HCV subtype 4d.
Phylogenetic analysis of HCV nucleotide sequences from subjects who received ombitasvir/paritaprevir/ritonavir plus ribavirin for 12 weeks in the clinical study AGATE-I, identified 11 HCV genotype 4 subtypes (4a, 4c, 4d, 4e, 4f, 4k, 4n, 4p, 4q, 4r, 4t). Most subjects were infected with either subtype 4a (58%) or 4d (20%); 1 to 2 subjects were infected with each of the other genotype 4 subtypes. Two subjects who experienced virologic failure in AGATE-I were infected with HCV subtype 4a or 4d.
Baseline HCV polymorphisms are not expected to impact the likelihood of achieving SVR when TECHNIVIE is used as recommended to treat HCV genotype 4 infected patients, based on the low virologic failure rates observed in PEARL-I and AGATE-I, although limited data are available for genotype 4 subtypes other than 4a and 4d.
Cross-resistance may occur among NS5A inhibitors and among NS3/4A protease inhibitors within each individual class. The impact of prior ombitasvir or paritaprevir treatment experience on the efficacy of other NS5A inhibitors or NS3/4A protease inhibitors has not been studied. Similarly, the efficacy of TECHNIVIE has not been studied in subjects who have failed prior treatment with another NS5A inhibitor, NS3/4A protease inhibitor, or NS5B inhibitor.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Ombitasvir was not carcinogenic in a 6-month transgenic mouse study up to the highest dose tested (150 mg per kg per day). Similarly, ombitasvir was not carcinogenic in a 2-year rat study up to the highest dose tested (30 mg per kg per day), resulting in ombitasvir exposures approximately 16-fold higher than those in humans at 25 mg.
Ombitasvir and its major inactive human metabolites (M29, M36) were not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes and in vivo mouse micronucleus assays.
Paritaprevir, ritonavir was not carcinogenic in a 6-month transgenic mouse study up to the highest dose tested (300/30 mg per kg per day). Similarly, paritaprevir, ritonavir was not carcinogenic in a 2-year rat study up to the highest dose tested (300/30 mg per kg per day), resulting in paritaprevir exposures approximately 11-fold higher than those in humans at 150 mg.
Paritaprevir was positive in an in vitro chromosome aberration test using human lymphocytes. Paritaprevir was negative in a bacterial mutation assay, and in two in vivo genetic toxicology assays (rat bone marrow micronucleus and rat liver Comet tests).
TECHNIVIE is administered with ribavirin. Refer to the prescribing information for ribavirin for information on carcinogenesis and mutagenesis.
Ombitasvir had no effects on embryo-fetal viability or on fertility when evaluated in mice up to the highest dose of 200 mg per kg per day. Ombitasvir exposures at this dose were approximately 26-fold the exposure in humans at the recommended clinical dose.
Paritaprevir, ritonavir had no effects on embryo-fetal viability or on fertility when evaluated in rats up to the highest dose of 300/30 mg per kg per day. Paritaprevir exposures at this dose were approximately 3- to 8-fold the exposure in humans at the recommended clinical dose.
TECHNIVIE is administered with ribavirin. Refer to the prescribing information for ribavirin for information on Impairment of Fertility.
14 CLINICAL STUDIES
14.1 Description of Clinical Trials
The efficacy and safety of TECHNIVIE was evaluated in two clinical trials in subjects with genotype 4 (GT4) chronic hepatitis virus (HCV) infection. In both trials, TECHNIVIE was administered with food and weight based ribavirin (1000 mg per day for subjects weighing less than 75 kg or 1200 mg per day for subjects weighing greater than or equal to 75 kg).The primary endpoint in both trials was sustained virologic response defined as HCV RNA below the lower limit of quantification (<LLOQ) 12 weeks after the end of treatment (SVR12) using the COBAS TaqMan HCV test (version 2.0), for use with the High Pure System, which has an LLOQ of 25 IU per mL. Previous exposure to HCV direct-acting antivirals was prohibited.
14.2 Clinical Trial Results in Adults with Chronic GT4 HCV Infection without Cirrhosis
PEARL-I was a randomized, global, multicenter, open-label trial that enrolled 135 adults without cirrhosis who were either treatment-naïve or did not achieve a virologic response with prior treatment with pegylated interferon/ribavirin (pegIFN/RBV). Treatment-naïve subjects were randomized in a 1:1 ratio to receive one ombitasvir 25 mg tablet, three paritaprevir 50 mg tablets and one ritonavir 100 mg capsule once-daily with food with or without ribavirin for 12 weeks. PegIFN/RBV treatment-experienced subjects received were assigned to receive the same doses as treatment naïve subjects.
Subjects had a median age of 51 years (range: 19 to 70); 64% were treatment-naïve, 17% were prior pegIFN/RBV null responders; 7% were prior pegIFN/RBV partial responders, 13% were prior pegIFN/RBV relapsers; 65% were male; 9% were Black; 14% had a body mass index at least 30 kg/m2; 70% had baseline HCV RNA levels at least 800,000 IU/mL; 79% had IL28B (rs12979860) non-CC genotype; 7% had bridging fibrosis (F3).
Table 9 presents the SVR12 rates.
| Treatment outcome | Ombitasvir + Paritaprevir
+ Ritonavir with RBV for 12 weeks | Ombitasvir + Paritaprevir
+ Ritonavir for 12 weeks |
|
| Treatment-naïve | Treatment-experienced | Treatment-naïve | |
| % (n/N) | % (n/N) | % (n/N) | |
| Overall SVR12 | 100% (42/42) | 100% (49/49) | 91% (40/44) |
| Outcome for subjects without SVR12 | |||
| On-treatment VFa | 0% (0/42) | 0% (0/49) | 2% (1/44) |
| Relapseb | 0% (0/42) | 0% (0/49) | 5% (2/42) |
| Otherc | 0% (0/42) | 0% (0/49) | 2% (1/44) |
VF = virologic failure
|
|||
Among the 131 HCV genotype 4 infected subjects in PEARL-I who achieved SVR12, virologic response data at post-treatment week 24 were available from 129 subjects, and 129/129 (100%) subjects maintained their response through 24 weeks post-treatment (SVR24).
14.3 Clinical Trial Results in Adults with Chronic GT4 HCV Infection with Compensated Cirrhosis
AGATE-I was a global multicenter, open-label trial in 120 HCV genotype 4 infected adults with compensated cirrhosis who were either treatment-naïve or pegIFN/RBV treatment-experienced and were treated for 12 weeks (n=59) or 16 weeks (n=61) with TECHNIVIE given once daily with weight based RBV. TECHNIVIE was administered as coformulated ombitasvir, paritaprevir, ritonavir tablets.
Of the 59 subjects in the 12 week arm, median age was 56 years (range: 43 to 81); 51% were treatment-naïve, 29% were prior pegIFN/RBV null responders; 8% were prior pegIFN/RBV partial responders, 12% were prior pegIFN/RBV relapsers; 76% were male; 17% were Black; 29% had a body mass index of at least 30 kg/m2; 76% had baseline HCV RNA levels of at least 800,000 IU per mL; 86% had IL28B (rs12979860) non‑CC genotype; 12% had platelet counts of less than 90 x 109 per L; and 5% had albumin less than 3.5 mg per dL.
Table 10 presents the SVR12 rates for HCV genotype 4 infected subjects with compensated cirrhosis treated with TECHNIVIE with RBV for 12 weeks. Treatment with 16 weeks was not shown to increase SVR12 rates therefore, TECHNIVIE with RBV for 16 week arm is not presented in Table 10.
| Treatment outcome | TECHNIVIE with RBV for 12 Weeks |
| % (n/N) | |
| SVR12, % (n/N) | 97% (57/59) |
| Outcome for subjects without SVR12 | |
| On-treatment VFa | 2% (1/59) |
| Relapseb | 0 (0/57) |
| Otherc | 2% (1/59) |
|
|
16 HOW SUPPLIED/STORAGE AND HANDLING
TECHNIVIE is dispensed in a monthly carton for a total of 28 days of therapy. Each monthly carton contains four weekly cartons. Each weekly carton contains seven daily dose packs.
Each child resistant daily dose pack contains two TECHNIVIE tablets. The NDC number is 0074-3082-28.
TECHNIVIE is a pink-colored, film-coated, oblong, biconvex-shaped tablet debossed with “AV1” on one side. Each tablet contains 12.5 mg ombitasvir, 75 mg paritaprevir and 50 mg ritonavir.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients to review the Medication Guide for ribavirin [see Warnings and Precautions (5.3)].
Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
Inform patients that HBV reactivation can occur in patients coinfected with HBV during or after treatment of HCV infection. Advise patients to tell their healthcare provider if they have a history of hepatitis B virus infection [see Warnings and Precautions (5.1)].
Risk of ALT Elevations or Hepatic Decompensation and Failure
Inform patients to watch for early warning signs of liver inflammation or failure, such as fatigue, weakness, lack of appetite, nausea and vomiting, as well as later signs such as jaundice, onset of confusion, abdominal swelling, and discolored feces, and to consult their health care professional without delay if such symptoms occur [see Warnings and Precautions (5.2 and 5.3) and Adverse Reactions (6.1)].
Advise patients that extreme care must be taken to avoid pregnancy during treatment with TECHNIVIE with ribavirin and within 6 months of stopping ribavirin in both female patients and in female partners of male patients. Inform patients to notify their health care provider immediately in the event of a pregnancy [see Use in Specific Populations (8.1)].
Inform patients that TECHNIVIE may interact with some drugs; therefore, patients should be advised to report to their healthcare provider the use of any prescription, non-prescription medication or herbal products [see Contraindications (4), Warnings and Precautions (5.5) and Drug Interactions (7)].
Inform patients that contraceptives containing ethinyl estradiol are contraindicated with TECHNIVIE [see Contraindications (4) and Warnings and Precautions (5.3)].
Advise patients to take TECHNIVIE every day at the regularly scheduled time with a meal without regard to fat or calorie content [see Dosage and Administration (2.1)].
Inform patients that it is important not to miss or skip doses and to take TECHNIVIE for the duration that is recommended by the healthcare provider.
Advise patients not to remove tablets from the daily dose pack until they are ready to take them.
Manufactured by AbbVie Inc., North Chicago, IL 60064.
TECHNIVIE and NORVIR are trademarks of AbbVie Inc.
© 2019 AbbVie Inc. All rights reserved.
03-C074
| TECHNIVIE
ombitasvir and paritaprevir and ritonavir kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |

