TETRACAINE HYDROCHLORIDE- tetracaine hydrochloride solution
Lake Erie Medical DBA Quality Care Products LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5%
(Sterile)
Rx only
DESCRIPTION:
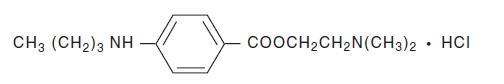
Tetracaine Hydrochloride is a sterile aqueous topical anesthetic ophthalmic solution. The active ingredient is represented by the chemical structure:
C15H24N2O2·HCI
Mol. wt. 300.83
Benzoic acid, 4-[butylamino]-, 2-[dimethylamino]ethyl ester, monohydrochloride.
EACH mL CONTAINS: ACTIVE: Tetracaine Hydrochloride 5 mg (0.5%); INACTIVES: Boric Acid, Potassium Chloride, Edetate Disodium and Purified Water. Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (3.7 - 6.0). PRESERVATIVE ADDED: Chlorobutanol 0.4%.
CLINICAL PHARMACOLOGY:
Topical anesthetics stabilize the neuronal membrane and prevent the initiation and transmission of nerve impulses, thereby effecting local anesthesia. The onset of anesthesia usually begins within 30 seconds and lasts a relatively short period of time.
INDICATIONS AND USAGE:
For procedures in which a rapid and short-acting topical ophthalmic anesthetic is indicated such as in tonometry, gonioscopy, removal of corneal foreign bodies, conjunctival scraping for diagnostic purposes, suture removal from the cornea, other short corneal and conjunctival procedures.
CONTRAINDICATIONS:
Should not be used by the patient without physician supervision, or in those persons showing hypersensitivity to any component of this preparation. This product should never be prescribed for the patient’s own use.
WARNINGS:
Prolonged use results in diminished duration of anesthesia and retarded healing. This may cause the drug to be used more frequently creating a “vicious circle.” Subsequent corneal infection and/or corneal opacification with accompanying permanent visual loss or corneal perforation may occur.
PRECAUTIONS:
FOR TOPICAL USE ONLY—NOT FOR INJECTION. To prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding area with the dropper tip. Patient should be advised not to touch or rub the eye(s) until the effect of the anesthetic has worn off.
ADVERSE REACTIONS:
Transient symptoms (signs) such as stinging, burning and conjunctival redness may occur. A rare, severe, immediate allergic cornea reaction has been reported, characterized by acute diffuse epithelial keratitis with filament formation and/or sloughing of large areas of necrotic epithelium, diffuse stromal edema, descemetitis and iritis.
DOSAGE AND ADMINISTRATION:
For tonometry and other procedures of short duration, instill one or two drops just prior to evaluation. For minor surgical procedures such as foreign body or suture removal, administer one to two drops every five to ten minutes for one to three instillations. For prolonged anesthesia as in cataract extraction, instill one or two drops in the eye(s) every five to ten minutes for three to five doses.
| TETRACAINE HYDROCHLORIDE
tetracaine hydrochloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lake Erie Medical DBA Quality Care Products LLC | 831276758 | relabel(49999-268) | |