Label: SODIUM THIOSULFATE injection, solution
- NDC Code(s): 60267-705-50
- Packager: Hope Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use the SODIUM THIOSULFATE INJECTION safely and effectively. See full prescribing information for SODIUM THIOSULFATE INJECTION.
Sodium Thiosulfate Injection, USP
Initial U.S. Approval: 1992INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- If clinical suspicion of cyanide poisoning is high, administer Sodium Thiosulfate Injection without delay and in conjunction with appropriate airway, ventilatory, and circulatory support. (2.1)
- The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222. (2.1)
Dosing:
Age Intravenous Dose of Sodium Nitrite and Sodium Thiosulfate Adults - 1.)
- Sodium Nitrite -10 mL of sodium nitrite at the rate of 2.5 to 5 mL/minute
- 2.)
- Sodium Thiosulfate - 50 mL of sodium thiosulfate immediately following administration of sodium nitrite.
Children - 1.)
- Sodium Nitrite - 0.2 mL/kg (6 mg/kg or 6-8 mL/m2 BSA) of sodium nitrite at the rate of 2.5 to 5 mL/minute not to exceed 10 mL
- 2.)
- Sodium Thiosulfate - 1 mL/kg of body weight (250 mg/kg or approximately 30-40 mL/m2 of BSA) not to exceed 50 mL total dose immediately following administration of sodium nitrite.
- Redosing: If signs of cyanide poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate. (2.2)
- Monitoring: Blood pressure must be monitored during treatment. (2.2)
- Sodium thiosulfate is chemically incompatible with hydroxocobalamin and should not be administered via the same intravenous line. (2.4)
DOSAGE FORMS AND STRENGTHS
Sodium Thiosulfate Injection consists of:
- One vial of sodium thiosulfate injection USP 12.5 grams/50 mL (250 mg/mL) (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Sulfites: Sodium thiosulfate may contain trace impurities of sodium sulfite (5.1)
ADVERSE REACTIONS
Most common adverse reactions are:
- Hypotension, headache, disorientation (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hope Pharmaceuticals at 1-800-755-9595 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Recommended Dosing
2.3 Recommended Monitoring
2.4 Incompatibility Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Sulfites
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Disease
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12. 2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Sodium Thiosulfate Injection is indicated for sequential use with sodium nitrite for the treatment of acute cyanide poisoning that is judged to be serious or life-threatening. When the diagnosis of cyanide poisoning is uncertain, the potential risks associated with Sodium Thiosulfate Injection should be carefully weighed against the potential benefits, especially if the patient is not in extremis.
-
2. DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- If clinical suspicion of cyanide poisoning is high, administer Sodium Thiosulfate Injection without delay.
- Comprehensive treatment of acute cyanide intoxication requires support of vital functions. Administration of sodium nitrite and sodium thiosulfate should be considered adjunctive to appropriate supportive therapies. Airway, ventilatory and circulatory support, and oxygen administration should not be delayed in order to administer sodium nitrite and sodium thiosulfate [see Warnings and Precautions (5.1)].
- The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222.
Identifying Patients with Cyanide Poisoning
Cyanide poisoning may result from inhalation, ingestion, or dermal exposure to various cyanide-containing compounds, including smoke from closed-space fires. Sources of cyanide poisoning include hydrogen cyanide and its salts, cyanogenic plants, aliphatic nitriles, and prolonged exposure to sodium nitroprusside.
The presence and extent of cyanide poisoning are often initially unknown. There is no widely available, rapid, confirmatory cyanide blood test. Treatment decisions must be made on the basis of clinical history and signs and symptoms of cyanide intoxication.
Table 1. Common Signs and Symptoms of Cyanide Poisoning Symptoms Signs - Headache
- Confusion
- Dyspnea
- Chest Tightness
- Nausea
- Altered Mental Status (e.g., confusion, disorientation)
- Seizures or Coma
- Mydriasis
- Tachypnea/Hyperpnea (early)
- Bradypnea/Apnea (late)
- Hypertension (early)/ Hypotension (late)
- Cardiovascular Collapse
- Vomiting
- Plasma Lactate Concentration ≥ 8 mmol/L
In some settings, panic symptoms including tachypnea and vomiting may mimic early cyanide poisoning signs. The presence of altered mental status (e.g., confusion and disorientation) and/or mydriasis is suggestive of true cyanide poisoning although these signs can occur with other toxic exposures as well.
Smoke Inhalation
Not all smoke inhalation victims will have cyanide poisoning and may present with burns, trauma, and exposure to other toxic substances making a diagnosis of cyanide poisoning particularly difficult. Prior to administration of Sodium Thiosulfate Injection, smoke-inhalation victims should be assessed for the following:
- Exposure to fire or smoke in an enclosed area
- Presence of soot around the mouth, nose, or oropharynx
- Altered mental status
Although hypotension is highly suggestive of cyanide poisoning, it is only present in a small percentage of cyanide-poisoned smoke inhalation victims. Also indicative of cyanide poisoning is a plasma lactate concentration greater than or equal to 10 mmol/L (a value higher than that typically listed in the table of signs and symptoms of isolated cyanide poisoning because carbon monoxide associated with smoke inhalation also contributes to lactic acidemia). If cyanide poisoning is suspected, treatment should not be delayed in order to obtain a plasma lactate concentration.
Use with Other Cyanide Antidotes
The safety of administering other cyanide antidotes simultaneously with Sodium Thiosulfate Injection has not been established. If a decision is made to administer another cyanide antidote with Sodium Thiosulfate Injection, these drugs should not be administered concurrently in the same intravenous (IV) line. [see Dosage and Administration (2.2)]
2.2 Recommended Dosing
Sodium Nitrite Injection and Sodium Thiosulfate Injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute life-threatening cyanide poisoning has been established. Sodium nitrite should be administered first, followed immediately by sodium thiosulfate. Blood pressure must be monitored during infusion in both adults and children. The rate of infusion should be decreased if significant hypotension is noted.
Age Intravenous Dose of Sodium Nitrite and Sodium Thiosulfate Adults - 1.)
- Sodium Nitrite -10 mL of sodium nitrite at the rate of 2.5 to 5 mL/minute
- 2.)
- Sodium Thiosulfate - 50 mL of sodium thiosulfate immediately following administration of sodium nitrite.
Children - 1.)
- Sodium Nitrite -0.2 mL/kg (6 mg/kg or 6-8 mL/m2 BSA) of sodium nitrite at the rate of 2.5 to 5 mL/minute not to exceed 10 mL
- 2.)
- Sodium Thiosulfate - 1 mL/kg of body weight (250 mg/kg or approximately 30-40 mL/m2 of BSA) not to exceed 50 mL total dose immediately following administration of sodium nitrite.
NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate.
In adult and pediatric patients with known anemia, it is recommended that the dosage of sodium nitrite should be reduced proportionately to the hemoglobin concentration.
Visually inspect all parenteral drug products for particulate matter and discoloration prior to administration.
2.3 Recommended Monitoring
Patients should be monitored for at least 24-48 hours after Sodium Thiosulfate Injection administration for adequacy of oxygenation and perfusion and for recurrent signs and symptoms of cyanide toxicity. When possible, obtain hemoglobin/hematocrit when treatment is initiated. Measurements of oxygen saturation using standard pulse oximetry and calculated oxygen saturation values based on measured PO2 are unreliable in the presence of methemoglobinemia.
2.4 Incompatibility Information
Chemical incompatibility has been reported between Sodium Thiosulfate Injection and hydroxocobalamin and these drugs should not be administered simultaneously through the same IV line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same IV line as described in Dosage and Administration.
Simultaneous administration of Sodium Thiosulfate Injection and blood products (whole blood, packed red cells, platelet concentrate and/or fresh frozen plasma) through the same intravenous line is not recommended. However, blood products and Sodium Thiosulfate Injection can be administered simultaneously using separate intravenous lines (preferably on contralateral extremities, if peripheral lines are being used).
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
There have been no controlled clinical trials conducted to systematically assess the adverse events profile of sodium thiosulfate.
The medical literature has reported the following adverse events in association with sodium thiosulfate administration. These adverse events were not reported in the context of controlled trials or with consistent monitoring and reporting methodologies for adverse events. Therefore, frequency of occurrence of these adverse events cannot be assessed.
Cardiovascular system: hypotension
Central nervous system: headache, disorientation
Gastrointestinal system: nausea, vomiting
Hematological: prolonged bleeding time
Body as a Whole: salty taste in mouth, warm sensation over body
In humans, rapid administration of concentrated solutions or solutions not freshly prepared, and administration of large doses of sodium thiosulfate have been associated with a higher incidence of nausea and vomiting. However, administration of 0.1 g sodium thiosulfate per pound up to a maximum of 15 g in a 10-15% solution over 10-15 minutes was associated with nausea and vomiting in 7 of 26 patients without concomitant cyanide intoxication.
In a series of 11 human subjects, a single intravenous infusion of 50 mL of 50% sodium thiosulfate was associated with increases in clotting time 1-3 days after administration. However, no significant changes were observed in other hematological parameters.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Sodium Thiosulfate Injection use in pregnant women to establish a drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the pregnant woman and fetus associated with untreated cyanide poisoning (see Clinical Considerations). Therefore, if a pregnant woman has known or suspected cyanide poisoning, Sodium Thiosulfate Injection for sequential use with sodium nitrite is recommended [see Indications and Usage (1)]. In published animal studies, no evidence of embryotoxicity or malformations was reported when sodium thiosulfate was administered during organogenesis to pregnant mice, rats, hamsters, or rats at 0.2 to 0.9 times the human daily dose of 12.5 g for cyanide poisoning. The studies did not test doses that were comparable to the human dose for cyanide poisoning (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data:
No malformations or evidence of embryo-fetal toxicity were noted when pregnant mice, rats, hamsters, or rabbits were administered oral doses of sodium thiosulfate of up to 550, 400, 400, or 580 mg/kg, respectively during organogenesis (0.2, 0.3, 0.26, and 0.9 times the human dose of 12.5 g/60 kg person for cyanide poisoning based on body surface area).
Published studies suggest that treatment with sodium thiosulfate ameliorates the teratogenic effects of maternal cyanide poisoning in hamsters.
8.2 Lactation
Risk Summary
There are no data on the presence of sodium thiosulfate in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Cyanide and thiocyanate (which is formed when sodium thiosulfate combines with cyanide) are present in human milk. Because of the potential for serious adverse reactions in the breastfed infant, breastfeeding is not recommended during treatment with Sodium Thiosulfate Injection. There are no data to determine when breastfeeding may be safely restarted following the administration of Sodium Thiosulfate Injection.
8.4 Pediatric Use
There are case reports in the medical literature of sodium nitrite in conjunction with sodium thiosulfate being administered to pediatric patients with cyanide poisoning; however, there have been no clinical studies to evaluate the safety or efficacy of sodium thiosulfate in the pediatric population. As for adult patients, dosing recommendations for pediatric patients have been based on theoretical calculations of antidote detoxifying potential, extrapolation from animal experiments, and a small number of human case reports.
8.5 Geriatric Use
Sodium thiosulfate is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Disease
Sodium thiosulfate is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
10 OVERDOSAGE
There is limited information about the effects of large doses of sodium thiosulfate in humans. Oral administration of 3 g sodium thiosulfate per day for 1-2 weeks in humans resulted in reductions in room air arterial oxygen saturation to as low as 75%, which was due to a rightward shift in the oxygen hemoglobin dissociation curve. The subjects returned to baseline oxygen saturations 1 week after discontinuation of sodium thiosulfate. A single intravenous administration of 20 mL of 10% sodium thiosulfate reportedly did not change oxygen saturations.
-
11 DESCRIPTION
Sodium thiosulfate has the chemical name thiosulfuric acid, disodium salt, pentahydrate. The chemical formula is Na2S2O3∙ 5H2O and the molecular weight is 248.17. The structural formula is:
Structure of Sodium Thiosulfate Pentahydrate
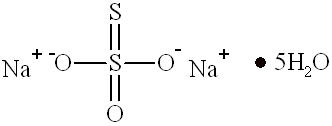
Sodium Thiosulfate Injection is a cyanide antidote which contains one 50 mL glass vial containing a 25% solution of sodium thiosulfate injection.
Sodium thiosulfate injection is a sterile aqueous solution and is intended for intravenous injection. Each vial contains 12.5 grams of sodium thiosulfate in 50 mL solution (250 mg/mL). Each mL also contains 2.8 mg boric acid and 4.4 mg of potassium chloride. The pH of the solution is adjusted with boric acid and/or sodium hydroxide. Sodium thiosulfate injection is a clear solution with a pH between 7.5 and 9.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Exposure to a high dose of cyanide can result in death within minutes due to the inhibition of cytochrome oxidase resulting in arrest of cellular respiration. Specifically, cyanide binds rapidly with cytochrome a3, a component of the cytochrome c oxidase complex in mitochondria. Inhibition of cytochrome a3 prevents the cell from using oxygen and forces anaerobic metabolism, resulting in lactate production, cellular hypoxia and metabolic acidosis. In massive acute cyanide poisoning, the mechanism of toxicity may involve other enzyme systems as well.
The synergy resulting from treatment of cyanide poisoning with the combination of sodium nitrite and sodium thiosulfate is the result of differences in their primary mechanisms of action as antidotes for cyanide poisoning.
Sodium Nitrite
Sodium nitrite is thought to exert its therapeutic effect by reacting with hemoglobin to form methemoglobin, an oxidized form of hemoglobin incapable of oxygen transport but with high affinity for cyanide. Cyanide preferentially binds to methemoglobin over cytochrome a3, forming the nontoxic cyanomethemoglobin. Methemoglobin displaces cyanide from cytochrome oxidase, allowing resumption of aerobic metabolism. The chemical reaction is as follows:
NaNO2 + Hemoglobin → Methemoglobin
HCN + Methemoglobin → Cyanomethemoglobin
Vasodilation has also been cited to account for at least part of the therapeutic effect of sodium nitrite. It has been suggested that sodium nitrite-induced methemoglobinemia may be more efficacious against cyanide poisoning than comparable levels of methemoglobinemia induced by other oxidants. Also, sodium nitrite appears to retain some efficacy even when the formation of methemoglobin is inhibited by methylene blue.
Sodium Thiosulfate
The primary route of endogenous cyanide detoxification is by enzymatic transulfuration to thiocyanate (SCN-), which is relatively nontoxic and readily excreted in the urine. Sodium thiosulfate is thought to serve as a sulfur donor in the reaction catalyzed by the enzyme rhodanese, thus enhancing the endogenous detoxification of cyanide in the following chemical reaction:
Rhodanese Na2S2O3 + CN- → SCN- + Na2SO3. 12. 2 Pharmacodynamics
In dogs, pretreatment with sodium thiosulfate to achieve a steady state level of 2 μmol/mL increased the rate of conversion of cyanide to thiocyanate over 30-fold.
12.3 Pharmacokinetics
Sodium Thiosulfate
Thiosulfate taken orally is not systemically absorbed. Most of the thiosulfate is oxidized to sulfate or is incorporated into endogenous sulphur compounds; a small proportion is excreted through the kidneys. Approximately 20-50% of exogenously administered thiosulfate is eliminated unchanged via the kidneys. After an intravenous injection of 1 g sodium thiosulfate in patients, the reported serum thiosulfate half-life was approximately 20 minutes. However, after an intravenous injection of a substantially higher dose of sodium thiosulfate (150 mg/kg, that is, 9 g for 60 kg body weight) in normal healthy men, the reported elimination half-life was 182 minutes.
Cyanide
The apparent terminal elimination half life and volume of distribution of cyanide, in a patient treated for an acute cyanide poisoning with sodium nitrite and sodium thiosulfate administration, have been reported to be 19 hours and 0.41 L/kg, respectively. Additionally, an initial elimination half life of cyanide has been reported to be approximately 1-3 hours.
Thiocyanate
After detoxification, in healthy subjects, thiocyanate is excreted mainly in the urine at a rate inversely proportional to creatinine clearance. In healthy subjects, the elimination half-life and volume of distribution of thiocyanate have been reported to be 2.7 days and 0.25 L/kg, respectively. However, in subjects with renal insufficiency the reported elimination half life is approximately 9 days.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals have not been performed to evaluate the potential carcinogenicity of sodium thiosulfate.
Mutagenesis:
The mutagenic potential of sodium thiosulfate has been examined in the in vitro Bacterial Reverse Mutation Assay (Ames Assay). Sodium thiosulfate was not mutagenic in the absence of metabolic activation in S. typhimurium strains TA98, TA100, TA1535, TA537, or TA1538. Sodium thiosulfate was not mutagenic in the presence of metabolic activation in strains TA 98, TA1535, TA1537, TA1538 or E. coli strain WP2.
Sodium thiosulfate was negative for clastogenic potential in the in vivo mammalian erythrocyte micronucleus assay in rats.
-
14 CLINICAL STUDIES
Human Data
The human data supporting the use of sodium thiosulfate for cyanide poisoning consists primarily of published case reports. There are no randomized controlled clinical trials. Nearly all the human data describing the use of sodium thiosulfate report its use in conjunction with sodium nitrite. Dosing recommendations for humans have been based on theoretical calculations of antidote detoxifying potential, extrapolation from animal experiments, and a small number of human case reports.
There have been no human studies to prospectively and systematically evaluate the safety of sodium thiosulfate or sodium nitrite in humans. Available human safety information is based largely on anecdotal case reports and case series of limited scope.
Animal Data (Cyanide Poisoning)
THE EFFECTIVENESS OF SODIUM THIOSULFATE AND SODIUM NITRITE FOR THE TREATMENT OF ACUTE CYANIDE POISONING HAS NOT BEEN STUDIED IN HUMANS IN ADEQUATE AND WELL-CONTROLLED CLINICAL TRIALS BECAUSE INDUCING THE CONDITION IN HUMANS TO STUDY THE DRUG'S EFFICACY IS NOT ETHICAL.
Due to the extreme toxicity of cyanide, experimental evaluation of treatment efficacy has predominantly been completed in animal models. The efficacy of sodium thiosulfate treatment alone to counteract the toxicity of cyanide was initially reported in 1895 by Lang. The efficacy of amyl nitrite treatment in cyanide poisoning of the dog model was first reported in 1888 by Pedigo. Further studies in the dog model, which demonstrated the utility of sodium nitrite as a therapeutic intervention, were reported in 1929 by Mladoveanu and Gheorghiu. However, Hugs and Chen et al. independently reported upon the superior efficacy of the combination of sodium nitrite and sodium thiosulfate in 1932-1933. Treatment consisted of intravenously administered 22.5 mg/kg (half the lethal dose) sodium nitrite or 1 g/kg sodium thiosulfate alone or in sequence immediately after subcutaneous injection of sodium cyanide into dogs over a range of doses. Subsequent doses of 10 mg/kg sodium nitrite and/or 0.5 g/kg sodium thiosulfate were administered when clinical signs or symptoms of poisoning persisted or reappeared. Either therapy administered alone increased the dose of sodium cyanide required to cause death, and when administered together, sodium nitrite and sodium thiosulfate resulted in a synergistic effect in raising the lethal dose of sodium cyanide. The combined therapy appeared to have reduced efficacy when therapy was delayed until signs of poisoning (e.g. convulsions) appeared; however, other investigators have reported survival in dogs that were administered antidotal treatment after respiratory arrest had occurred.
Animal studies conducted in other species (e.g., rat, guinea pig, sheep, pigeon and cat) have also supported a synergistic effect of intravenous sodium nitrite and sodium thiosulfate in the treatment of cyanide poisoning.
While intravenous injection of sodium nitrite and sodium thiosulfate was effective in reversing the effects of lethal doses of cyanide in dogs, intramuscular injection of sodium nitrite, with or without sodium thiosulfate, was found not to be effective in the same setting.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Sodium Thiosulfate Injection is indicated for cyanide poisoning and in this setting, patients will likely be unresponsive or may have difficulty in comprehending counseling information.
Monitoring
Where feasible, patients should be informed of the need for close monitoring of blood pressure and oxygenation.
Lactation
Advise women that breastfeeding is not recommended during treatment with Sodium Thiosulfate Injection [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
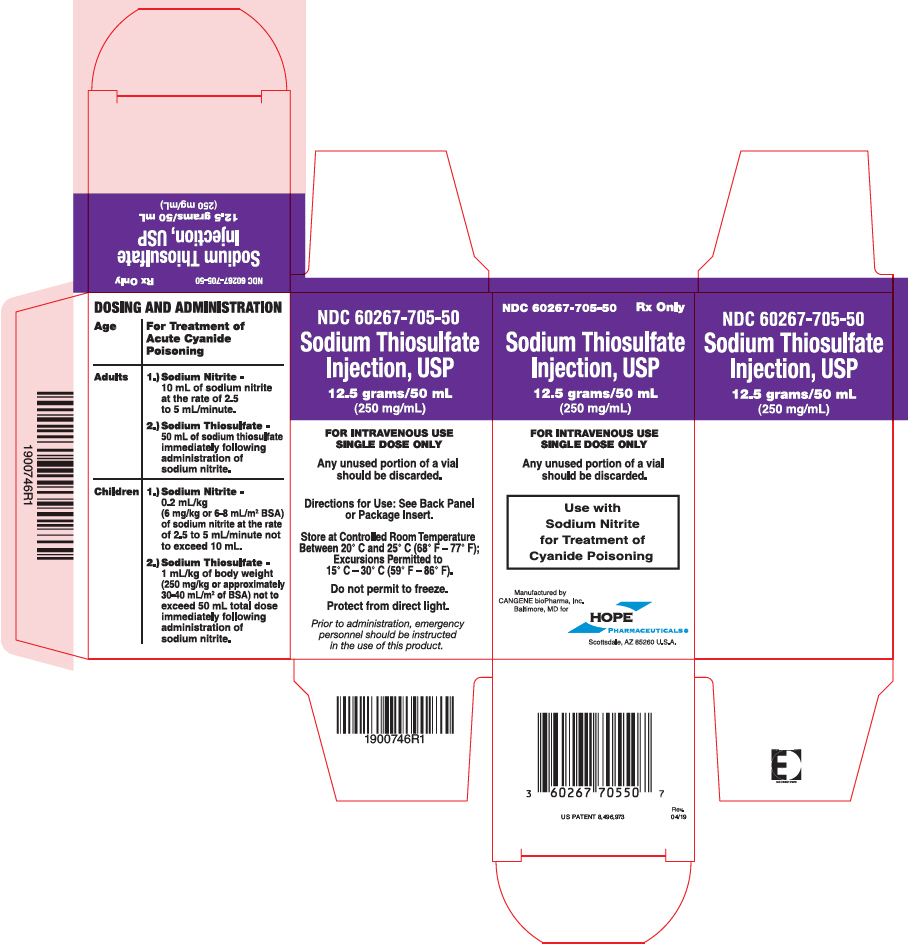
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
NDC 60267-705-50
Rx OnlySodium Thiosulfate
Injection, USP12.5 grams/50 mL
(250 mg/mL)FOR INTRAVENOUS USE
SINGLE DOSE ONLYAny unused portion of a vial
should be discarded.Use with
Sodium Nitrite
for Treatment of
Cyanide PoisoningManufactured by
CANGENE bioPharma, Inc.
Baltimore, MD forHOPE
PHARMACEUTICALS ®
Scottsdale, AZ 85260 U.S.A.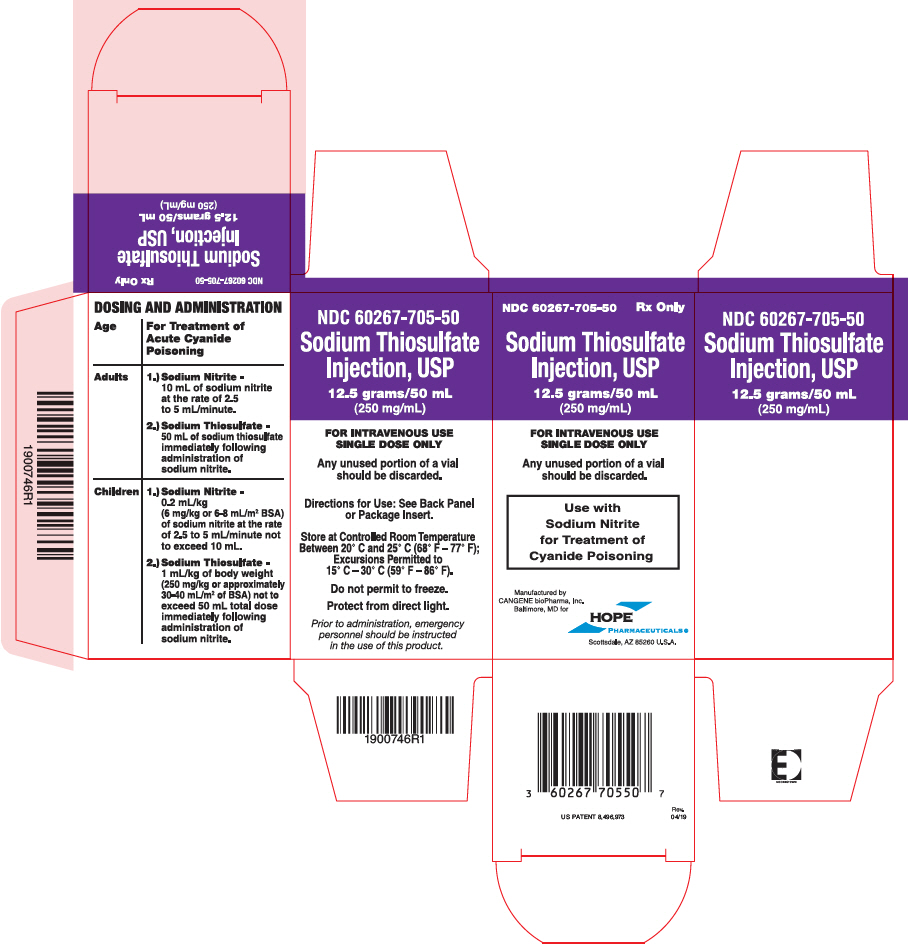
-
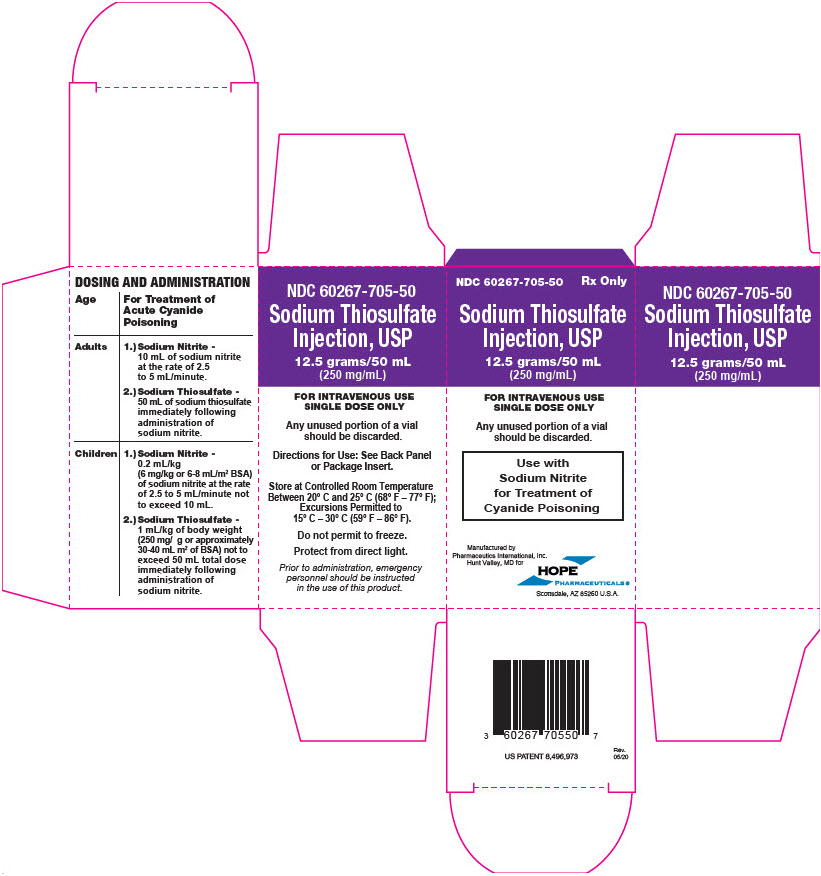
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton - Pharmaceutics International, Inc.
NDC 60267-705-50
Rx OnlySodium Thiosulfate
Injection, USP12.5 grams/50 mL
(250 mg/mL)FOR INTRAVENOUS USE
SINGLE DOSE ONLYAny unused portion of a vial
should be discarded.Use with
Sodium Nitrite
for Treatment of
Cyanide PoisoningManufactured by
Pharmaceutics International, Inc.
Hunt Valley, MD forHOPE
PHARMACEUTICALS ®
Scottsdale, AZ 85260 U.S.A.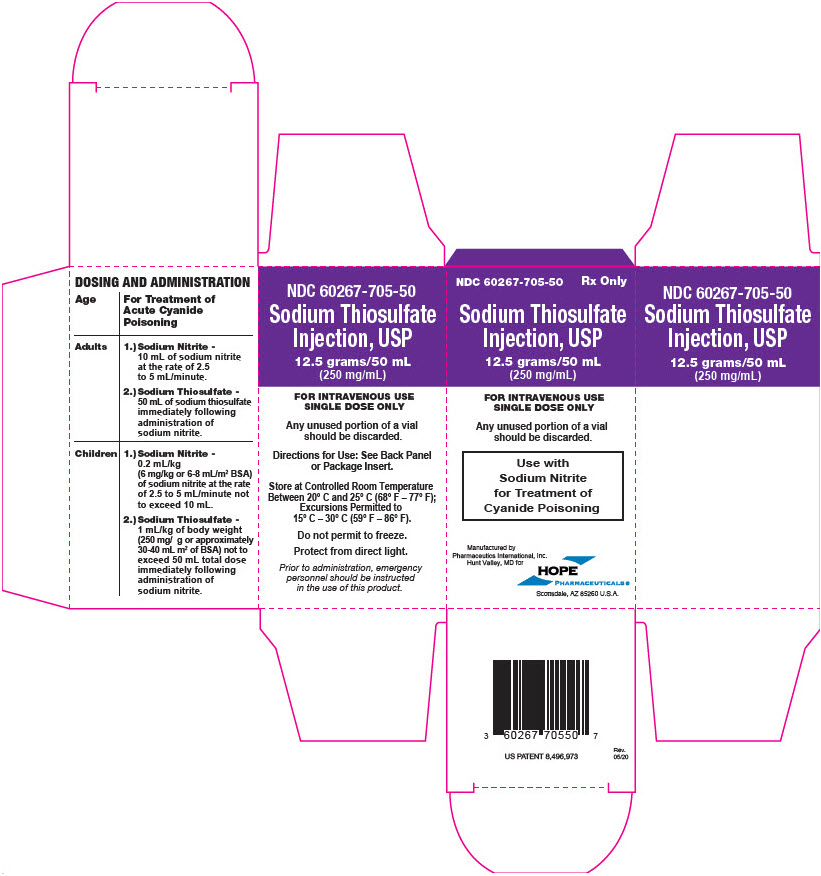
-
INGREDIENTS AND APPEARANCE
SODIUM THIOSULFATE
sodium thiosulfate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60267-705 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Thiosulfate (UNII: HX1032V43M) (THIOSULFATE ION - UNII:LLT6XV39PY) Sodium Thiosulfate 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength Potassium Chloride (UNII: 660YQ98I10) 4.4 mg in 1 mL Boric Acid (UNII: R57ZHV85D4) 2.8 mg in 1 mL Water (UNII: 059QF0KO0R) Sodium Hydroxide (UNII: 55X04QC32I) Nitrogen (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60267-705-50 50 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 02/14/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203923 02/14/2012 Labeler - Hope Pharmaceuticals (015227945) Establishment Name Address ID/FEI Business Operations Curia Wisconsin, Inc. 004670501 API MANUFACTURE(60267-705) Establishment Name Address ID/FEI Business Operations Cangene Biopharma, Inc. 050783398 MANUFACTURE(60267-705) Establishment Name Address ID/FEI Business Operations Sterling Wisconsin LLC 961717936 API MANUFACTURE(60267-705) Establishment Name Address ID/FEI Business Operations Curia New York, Inc. 124193793 API MANUFACTURE(60267-705) Establishment Name Address ID/FEI Business Operations Pharmaceutics International, Inc. 049185696 MANUFACTURE(60267-705)