Label: HEMORRHOID AND FISSURE DR. BUTLER- lidocaine 4.00, phenylephrine hydrochloride 0.25 ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 70942-023-01, 70942-023-11 - Packager: Beyond Health P.A.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
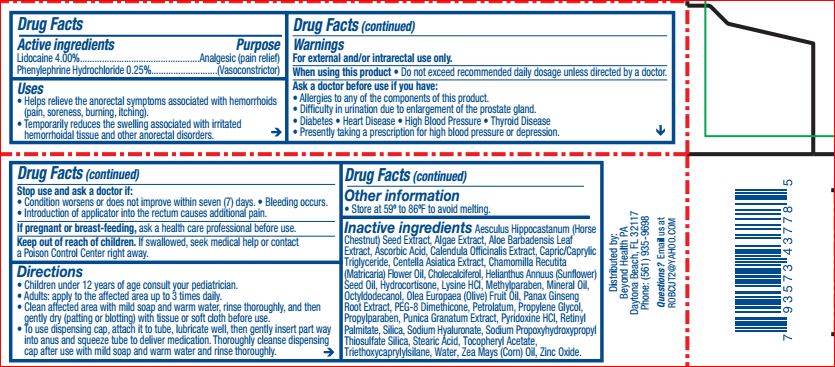
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external and/or intrarectal use only.
When using this product do not exceed recommended daily dosage unless directed by a doctor.
Ask a doctor before use if you have allergies to any of the components of this product. Difficulty in urination due to enlargement of the prostate gland. Diabetes, heart disease, high blood pressure, thyroid disease. Presently taking a prescription for high blood pressure or depression.
Stop use and ask a doctor if condition worsens or does not improve within seven days, bleeding occurs. Introduction of applicator into the rectum causes additional pain.
If pregnant or breastfeeding, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Children under 12 years of age consult your pediatrician.
Adults: apply to affected area up to 3 times daily.
Clean affected area with mild soap and warm water, rinse thoroughly, and then gently dry (patting or blotting) with tissue or soft cloth before use.
To use dispensing cap, attach it to tube, lubricate well then gently insert part way into anus and squeeze tube to deliver medication. Thoroughly cleanse dispensing cap after use with mild soap and warm water and rinse thoroughly.
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMORRHOID AND FISSURE DR. BUTLER
lidocaine 4.00, phenylephrine hydrochloride 0.25 ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70942-023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE 0.25 g in 100 g Inactive Ingredients Ingredient Name Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) ALOE (UNII: V5VD430YW9) ASCORBIC ACID (UNII: PQ6CK8PD0R) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) TRICAPRYLIN (UNII: 6P92858988) CENTELLA ASIATICA (UNII: 7M867G6T1U) CHOLECALCIFEROL (UNII: 1C6V77QF41) SUNFLOWER OIL (UNII: 3W1JG795YI) HYDROCORTISONE (UNII: WI4X0X7BPJ) LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) OCTYLDODECANOL (UNII: 461N1O614Y) OLIVE OIL (UNII: 6UYK2W1W1E) ASIAN GINSENG (UNII: CUQ3A77YXI) PEG-8 DIMETHICONE (UNII: GIA7T764OD) PETROLATUM (UNII: 4T6H12BN9U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) PUNICA GRANATUM ROOT BARK (UNII: CLV24I3T1D) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM PROPOXYHYDROXYPROPYL THIOSULFATE SILICA (UNII: 208G222332) STEARIC ACID (UNII: 4ELV7Z65AP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) CORN OIL (UNII: 8470G57WFM) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70942-023-01 1 in 1 CARTON 08/28/2007 1 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:70942-023-11 1 in 1 CARTON 08/27/2019 2 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 08/28/2007 Labeler - Beyond Health P.A. (026781064) Registrant - Derma Care Research Labs (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs 116817470 manufacture(70942-023)