Label: TOPCARE HEMORRHOIDAL- cocoa butter, phenylephrine hcl suppository
- NDC Code(s): 36800-279-53, 36800-279-62
- Packager: Topco Associates LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
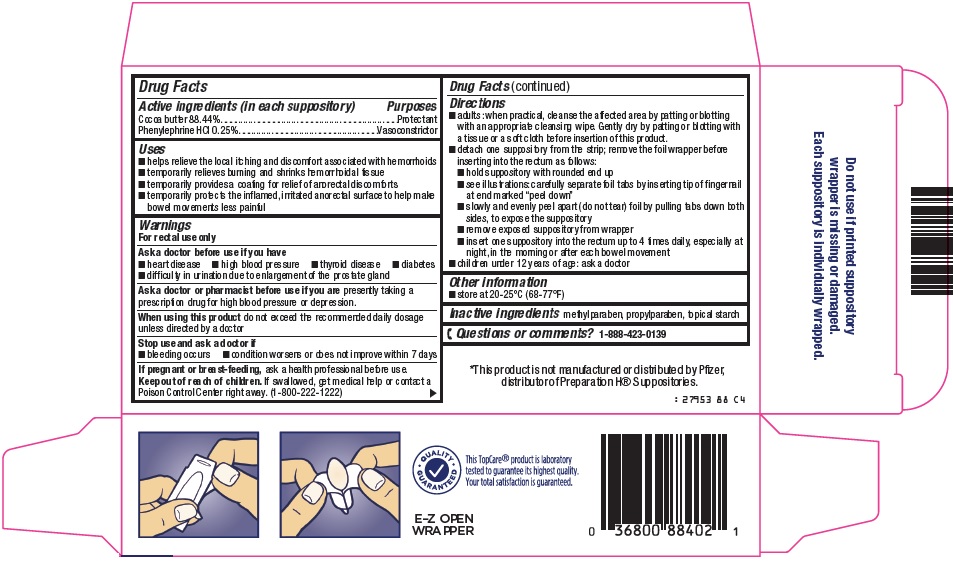
- Active ingredients (in each suppository)
- Purposes
-
Uses
- •
- helps relieve the local itching and discomfort associated with hemorrhoids
- •
- temporarily relieves burning and shrinks hemorrhoidal tissue
- •
- temporarily provides a coating for relief of anorectal discomforts
- •
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
-
Warnings
For rectal use only
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression.
-
Directions
- •
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before insertion of this product.
- •
- detach one suppository from the strip; remove the foil wrapper before inserting into the rectum as follows:
- •
- hold suppository with rounded end up
- •
- see illustrations: carefully separate foil tabs by inserting tip of fingernail at end marked “peel down”
- •
- slowly and evenly peel apart (do not tear) foil by pulling tabs down both sides, to expose the suppository
- •
- remove exposed suppository from wrapper
- •
- insert one suppository into the rectum up to 4 times daily, especially at night, in the morning or after each bowel movement
- •
- children under 12 years of age: ask a doctor
- Inactive ingredients
-
Package/Label Principal Display Panel
TopCare ® health
COMPARE TO PREPARATION H® SUPPOSITORIES ACTIVE INGREDIENTS
NIGHTTIME RELIEF
Hemorrhoidal Suppositories
● Reduces Internal Swelling. Soothes and Protects
● Prompt Soothing Relief from Painful Burning, Itching and Discomfort
● Shrinks Swollen Hemorrhoidal Tissue
● Protects Irritated Tissue
12 SUPPOSITORIES
E-Z OPEN WRAPPER
-
INGREDIENTS AND APPEARANCE
TOPCARE HEMORRHOIDAL
cocoa butter, phenylephrine hcl suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-279 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COCOA BUTTER (UNII: 512OYT1CRR) (COCOA BUTTER - UNII:512OYT1CRR) COCOA BUTTER 2211 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 6.5 mg Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color YELLOW (light) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-279-62 24 in 1 CARTON 11/03/2014 03/05/2019 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:36800-279-53 12 in 1 CARTON 11/03/2014 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 11/03/2014 Labeler - Topco Associates LLC (006935977)