NOCTIVA- desmopressin acetate spray, metered
Avadel Specialty Pharmaceuticals, LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NOCTIVA™ safely and effectively. See full prescribing information for NOCTIVA.
NOCTIVA (desmopressin acetate) nasal spray, for intranasal use Initial U.S. Approval: 1978 WARNING: HYPONATREMIASee full prescribing information for complete boxed warning.
INDICATIONS AND USAGEDOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSPreservative-free nasal spray delivering 0.83 mcg of desmopressin acetate (equivalent to 0.75 mcg desmopressin) or 1.66 mcg of desmopressin acetate (equivalent to 1.5 mcg desmopressin) in each spray (0.1 mL). (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSCommon adverse reactions in clinical trials (incidence >2%) included nasal discomfort, nasopharyngitis, nasal congestion, sneezing, hypertension/blood pressure increased, back pain, epistaxis, bronchitis, and dizziness. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Avadel at 1-877-638-4579 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSMonitor serum sodium more frequently when NOCTIVA is concomitantly used with drugs that may cause water retention and increase the risk for hyponatremia (e.g., tricyclic antidepressants, selective serotonin reuptake inhibitors, chlorpromazine, opiate analgesics, nonsteroidal anti-inflammatories, lamotrigine, and carbamazepine). (7.2) USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 12/2017 |
FULL PRESCRIBING INFORMATION
WARNING: HYPONATREMIA
- NOCTIVA can cause hyponatremia. Severe hyponatremia can be life-threatening, leading to seizures, coma, respiratory arrest, or death (5.1, 6.1).
- NOCTIVA is contraindicated in patients at increased risk of severe hyponatremia, such as patients with excessive fluid intake, illnesses that can cause fluid or electrolyte imbalances, and in those using loop diuretics or systemic or inhaled glucocorticoids (4, 5.1).
- Ensure serum sodium concentrations are normal before starting or resuming NOCTIVA. Measure serum sodium within 7 days and approximately 1 month after initiating therapy or increasing the dose, and periodically during treatment. More frequently monitor serum sodium in patients 65 years of age and older and in patients at increased risk of hyponatremia (2.3, 5.1).
- If hyponatremia occurs, NOCTIVA may need to be temporarily or permanently discontinued (5.1).
1 INDICATIONS AND USAGE
NOCTIVA is indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least 2 times per night to void.
Nocturnal polyuria was defined in the NOCTIVA clinical trials as nighttime urine production exceeding one-third of the 24-hour urine production.
Before starting NOCTIVA:
- Evaluate the patient for possible causes for the nocturia, including excessive fluid intake prior to bedtime, and optimize the treatment of underlying conditions that may be contributing to the nocturia.
- Confirm the diagnosis of nocturnal polyuria with a 24-hour urine collection, if one has not been obtained previously.
Limitation of Use:
NOCTIVA has not been studied in patients less than 50 years of age.
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration and Priming Instructions
- Only administer NOCTIVA intranasally. Do not shake the bottle.
- Prime NOCTIVA before using for the first time by pumping 5 actuations into the air away from the face.
- Re-prime by pumping 2 actuations into the air if the product has not been used for more than 3 days.
- If a dose is missed, do not double the dose at next use.
- Two sprays of NOCTIVA 0.83 mcg are not interchangeable with 1 spray of NOCTIVA 1.66 mcg. Prescribe the NOCTIVA nasal spray 1.66 mcg/0.1 mL bottle for patients who are or will be taking the 1.66 mcg dose.
2.2 Recommended Dosage
-
For patients younger than 65 years of age who are not at increased risk for hyponatremia:
- The recommended dose is 1 spray of NOCTIVA 1.66 mcg in either the left or right nostril approximately 30 minutes before going to bed.
-
For patients ≥65 years of age, or younger patients at increased risk for hyponatremia:
- The recommended starting dose is 1 spray of NOCTIVA 0.83 mcg in either the left or right nostril approximately 30 minutes before going to bed.
- After at least 7 days of treatment, the dose can be increased to 1.66 mcg, if needed, provided the serum sodium is within the normal range during treatment with the 0.83 mcg dose.
- The 0.83 mcg dose did not meet all prespecified efficacy endpoints in clinical trials but may have a lower risk of hyponatremia [see Adverse Reactions (6.1) and Clinical Studies (14)].
2.3 Monitoring of Serum Sodium Concentration
Check serum sodium concentrations:
- Prior to initiating or resuming NOCTIVA or increasing the dose. NOCTIVA is contraindicated in patients with hyponatremia or a history of hyponatremia [see Contraindications (4)].
- Within 7 days and approximately 1 month after initiating therapy or increasing the dose.
- Periodically during NOCTIVA therapy, as clinically appropriate. More frequent serum sodium monitoring is recommended for patients 65 years and older and for those at increased risk of hyponatremia.
If the patient develops hyponatremia, NOCTIVA may need to be temporarily or permanently discontinued, and treatment for the hyponatremia instituted, depending on the clinical circumstances, including the duration and severity of the hyponatremia [see Warnings and Precautions (5.1)].
3 DOSAGE FORMS AND STRENGTHS
- Preservative-free desmopressin acetate nasal spray.
- Each spray delivers 0.1 mL of NOCTIVA.
- Each spray of the 0.83 mcg/0.1 mL strength of desmopressin acetate contains 0.83 mcg of desmopressin acetate (equivalent to 0.75 mcg of desmopressin).
- Each spray of the 1.66 mcg/0.1 mL strength of desmopressin acetate contains 1.66 mcg of desmopressin acetate (equivalent to 1.5 mcg of desmopressin).
4 CONTRAINDICATIONS
NOCTIVA is contraindicated in patients with the following conditions due to an increased risk of severe hyponatremia:
- Hyponatremia or a history of hyponatremia [see Warnings and Precautions (5.1)]
- Polydipsia
- Primary nocturnal enuresis [see Use in Specific Populations (8.4)]
- Concomitant use with loop diuretics [see Warnings and Precautions (5.1)]
- Concomitant use with systemic or inhaled glucocorticoids [see Warnings and Precautions (5.1), Drug Interactions (7.1)]
- Renal impairment with an estimated glomerular filtration rate (eGFR) below 50 mL/min/1.73 m2[see Use in Specific Populations (8.6)]
- Known or suspected syndrome of inappropriate antidiuretic hormone (SIADH) secretion
- During illnesses that can cause fluid or electrolyte imbalance, such as gastroenteritis, salt-wasting nephropathies, or systemic infection
NOCTIVA is contraindicated in patients with the following conditions because fluid retention increases the risk of worsening the underlying condition:
- Congestive heart failure (New York Heart Association Class II to IV) [see Warnings and Precautions (5.2)]
- Uncontrolled hypertension
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hyponatremia
NOCTIVA can cause hyponatremia [see Boxed Warning and Adverse Reactions (6.1)]. Severe hyponatremia can be life-threatening if it is not promptly diagnosed and treated, leading to seizures, coma, respiratory arrest, or death.
NOCTIVA is contraindicated in patients at increased risk of severe hyponatremia, such as those with excessive fluid intake, those who have illnesses that can cause fluid or electrolyte imbalances, and in those using loop diuretics or systemic or inhaled glucocorticoids [see Boxed Warning, Contraindications (4), and Drug Interactions (7.1)].
Before starting or resuming NOCTIVA, ensure that the serum sodium concentration is normal. Consider the 0.83 mcg dose as the starting dose for patients who may be at risk for hyponatremia [see Dosage and Administration (2.3, 2.2) and Clinical Studies (14)].
When NOCTIVA is administered, fluid intake in the evening and nighttime hours should be moderated to decrease the risk of hyponatremia. Monitor the serum sodium concentration within 7 days and approximately 1 month of initiating NOCTIVA or increasing the dose, and periodically thereafter. The frequency of serum sodium monitoring should be based on the patient’s risk for hyponatremia. For example, more frequent monitoring is recommended for patients 65 years of age or older or those on concomitant medications that can increase the risk of hyponatremia, such as tricyclic antidepressants, selective serotonin reuptake inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), chlorpromazine, carbamazepine, and thiazide diuretics [see Drug Interactions (7.2)].
If hyponatremia occurs, NOCTIVA may need to be temporarily or permanently discontinued, and treatment for the hyponatremia instituted, depending on the clinical circumstances, including the duration and severity of the hyponatremia [see Dosage and Administration (2.3)].
5.2 Fluid Retention
NOCTIVA can cause fluid retention, which can worsen underlying conditions that are susceptible to volume status. Therefore, NOCTIVA is contraindicated in patients with New York Heart Association Class II to IV congestive heart failure or uncontrolled hypertension [see Contraindications (4)]. In addition, NOCTIVA is not recommended in patients at risk for increased intracranial pressure or those with a history of urinary retention, and should be used with caution (e.g., monitoring of volume status) in patients with New York Heart Association Class I congestive heart failure.
5.3 Concurrent Nasal Conditions
Discontinue NOCTIVA in patients with concurrent nasal conditions that may increase systemic absorption of NOCTIVA (e.g., atrophy of nasal mucosa, and acute or chronic rhinitis), because the increased absorption may increase the risk of hyponatremia. NOCTIVA can be resumed when these conditions resolve.
6 ADVERSE REACTIONS
The following adverse reaction is described elsewhere in the labeling:
- Hyponatremia [see Boxed Warning and Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Two randomized, double-blind, placebo-controlled, multicenter trials conducted in adults 50 years of age and older evaluated the efficacy and safety of NOCTIVA nasal spray compared to placebo. At baseline, 1045 patients treated with NOCTIVA 0.83 mcg or 1.66 mcg, or placebo, had nocturia due to nocturnal polyuria, wakening at least 2 times per night to urinate. Nocturnal polyuria was defined as nighttime urine production exceeding one-third of the 24-hour urine production. The mean age of the patients studied with nocturia due to nocturnal polyuria was 67 years with 42% between 50 and 64 years of age, and 58% aged 65 years and older. Fifty- seven percent were men and 43% were women. Caucasians comprised 79%, Blacks 12%, Hispanics 6%, and Asians 2% of the trial population.
During these trials, serious adverse reactions were reported in 2%, 2%, and 3% of patients with nocturia due to nocturnal polyuria treated with NOCTIVA 0.83 mcg, NOCTIVA 1.66 mcg, and placebo, respectively. There was one case of hyponatremia in the 1.66 mcg group and one case in the placebo group classified as serious adverse reactions.
Adverse Reactions Leading to Discontinuation
Among patients with nocturia due to nocturnal polyuria, the discontinuation rate due to adverse reactions was 4.0% with NOCTIVA 0.83 mcg, 4.4% with NOCTIVA 1.66 mcg, and 2.3% with placebo. Table 1 displays the most common adverse reactions leading to discontinuation in patients with nocturia due to nocturnal polyuria.
| Adverse Reactions | NOCTIVA
1.66 mcg (n=341) | NOCTIVA
0.83 mcg (n=354) | Placebo
(n=349) |
| Hyponatremia/Blood Sodium Decreased | 4 (1.2%) | 3 (0.9%) | 1 (0.3%) |
| Nasal Discomfort | 2 (0.6%) | 0 | 3 (0.9%) |
| Nasal Congestion | 2 (0.6%) | 0 | 0 |
| Atrial Fibrillation | 2 (0.6%) | 0 | 0 |
| Dizziness | 0 | 2 (0.6%) | 1 (0.3%) |
| Dysuria | 1 (0.3%) | 2 (0.6%) | 0 |
Most Common Adverse Reactions
Table 2 summarizes the most common adverse reactions reported by patients with nocturia due to nocturnal polyuria. This table shows adverse reactions reported in at least 2% of patients treated with NOCTIVA and at a higher incidence with the 1.66 mcg dose than with placebo.
| Adverse Reactions | NOCTIVA
1.66 mcg (n=341) | NOCTIVA
0.83 mcg (n=354) | Placebo
(n=349) |
| Nasal Discomfort | 20 (5.9%) | 12 (3.4%) | 17 (4.9%) |
| Nasopharyngitis | 13 (3.8%) | 8 (2.3%) | 10 (2.9%) |
| Nasal Congestion | 10 (2.9%) | 5 (1.4%) | 5 (1.4%) |
| Sneezing | 9 (2.6%) | 8 (2.3%) | 5 (1.4%) |
| Hypertension/Blood Pressure Increased | 9 (2.6%) | 6 (1.7%) | 4 (1.1%) |
| Back Pain | 8 (2.3%) | 4 (1.1%) | 3 (0.9%) |
| Epistaxis | 7 (2.1%) | 7 (2.0%) | 4 (1.1%) |
| Bronchitis | 7 (2.1%) | 3 (0.8%) | 3 (0.9%) |
| Dizziness | 6 (1.8%) | 7 (2.0%) | 5 (1.4%) |
No overall changes were observed in the safety profile during the open-label, uncontrolled extension trial with up to 126 weeks of follow-up.
Hyponatremia
Table 3 shows the incidence of serum sodium concentrations below the normal range reported in the 2 placebo-controlled trials.
| Serum Sodium
Concentrations (mmol/L) | NOCTIVA 1.66 mcg
(n=341) | NOCTIVA 0.83 mcg
(n=354) | Placebo (n=349) |
| 130-134 | 42 (12.3%) | 33 (9.3%) | 18 (5.2%) |
| 126-129 | 7 (2.1%) | 8 (2.3%) | 0 |
| ≤125 | 5 (1.5%) | 0 | 1 (0.3%) |
Of the 5 patients on NOCTIVA 1.66 mcg with serum sodium ≤125 mmol/L, all were 65 years of age or older. Four were men. The onset of the hyponatremia ranged from 6 days to 12 weeks after the start of dosing. Four of these patients were taking a concomitant systemic or inhaled glucocorticoid and 3 were taking an NSAID.
Sex
The incidence of hyponatremia with NOCTIVA was similar in men and women.
Age
Patients 65 years of age and older treated with NOCTIVA had a higher incidence of hyponatremia compared to those younger than 65 years of age (see Table 4).
| Serum Sodium
Concentrations (mmol/L) | NOCTIVA
1.66 mcg <65 years (n=146) | NOCTIVA
1.66 mcg ≥65 years (n=195) | NOCTIVA
0.83 mcg <65 years (n=148) | NOCTIVA
0.83 mcg ≥65 years (n=206) | Placebo
<65 years (n=144) | Placebo
≥65 years (n=205) |
| 130-134 | 14 (9.6%) | 28 (14.4%) | 8 (5.4%) | 25 (12.1%) | 7 (4.9%) | 11 (5.4%) |
| 126-129 | 0 | 7 (3.6%) | 2 (1.4%) | 6 (2.9%) | 0 | 0 |
| ≤125 | 0 | 5 (2.6%) | 0 | 0 | 0 | 1 (0.5%) |
7 DRUG INTERACTIONS
No specific pharmacokinetic studies were conducted to evaluate potential drug-drug interactions between NOCTIVA and other medications.
7.1 Drugs That May Cause Severe Hyponatremia
Concomitant use of NOCTIVA and loop diuretics or systemic or inhaled glucocorticoids is contraindicated because of the risk of severe hyponatremia [see Boxed Warning, Contraindications (4), and Warnings and Precautions (5.1)]. NOCTIVA can be started or resumed 3 days or 5 half-lives after the glucocorticoid is discontinued, whichever is longer.
7.2 Drugs That May Cause Water Retention
Monitor serum sodium more frequently in patients taking NOCTIVA concomitantly with medications that may cause water retention and increase the risk for hyponatremia (e.g., tricyclic antidepressants, selective serotonin reuptake inhibitors, chlorpromazine, opioid analgesics, NSAIDs, lamotrigine, and carbamazepine) [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with NOCTIVA use in pregnant women to inform any drug-associated risks. No adverse developmental outcomes were observed in animal reproduction studies with administration of desmopressin during organogenesis to pregnant rats and rabbits at doses approximately <1 and 31 times, respectively, the maximum recommended human dose based on nasal surface area (see Data).
NOCTIVA is not recommended for the treatment of nocturia in pregnant women. Nocturia is usually related to normal physiologic changes during pregnancy that do not require treatment with NOCTIVA.
In the U.S. general population, the estimated background rate of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
Desmopressin acetate did not cause fetal harm in teratology studies in rats and rabbits at doses from 0.05 to 10 mcg/kg/day, which is approximately <1 times (rat) and 31 times (rabbit) the maximum recommended human dose based on nasal surface area.
8.2 Lactation
Desmopressin is present in small amounts in human milk and is poorly absorbed orally by an infant. There is no information on the effects of desmopressin on the breastfed infant or on milk production. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for NOCTIVA and any potential adverse effects on the breastfed infant from NOCTIVA or from the underlying maternal condition.
8.4 Pediatric Use
NOCTIVA is contraindicated for the treatment of primary nocturnal enuresis because of reports of hyponatremic-related seizures in pediatric patients treated with other intranasal formulations of desmopressin. Studies of NOCTIVA have not been conducted in pediatric patients [see Contraindications (4)].
8.5 Geriatric Use
Patients 65 years and older treated with NOCTIVA had a higher incidence of hyponatremia compared to patients less than 65 years old treated with NOCTIVA [see Dosage and Administration (2.2), Warnings and Precautions (5.1), and Adverse Reactions (6.1)].
8.6 Renal Impairment
Desmopressin is mainly excreted in the urine. The area under the concentration-time curve (AUC) and terminal half-life of desmopressin in renally impaired patients with an eGFR below 50 mL/min/1.73 m2 is 3- to 4-fold greater than in patients with an eGFR above 50 mL/min/1.73 m2. Therefore, NOCTIVA is contraindicated in patients who have renal impairment with an eGFR below 50 mL/min/1.73 m2[see Contraindications (4) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Signs of overdose may include effects from hyponatremia such as seizure, altered mental status, cardiac arrhythmias, and worsening edema. Other signs of overdose may include oliguria and rapid weight gain due to fluid retention [see Warnings and Precautions (5.1)]. In case of overdosage, NOCTIVA should be discontinued immediately, serum sodium should be assessed, and appropriate medical treatment initiated.
11 DESCRIPTION
Desmopressin acetate is a synthetic analogue of 8-arginine vasopressin, an endogenous pituitary hormone also known as antidiuretic hormone (ADH). Its chemical name is 1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate, and its molecular weight is 1183.31. Its molecular formula is C48H68N14O14S2·3H2O. Its chemical structure is:
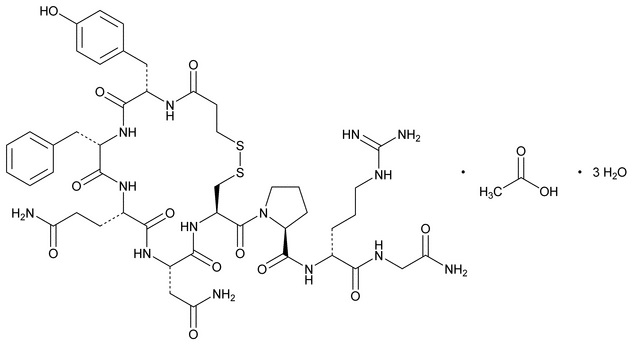
Desmopressin acetate is a white powder that is freely soluble in water. It is also soluble in alcohol and glacial acetic acid. NOCTIVA (desmopressin acetate) nasal spray is formulated for intranasal use as a milky white emulsion without preservatives at pH 5.5.
NOCTIVA is available as an oil-in-water emulsion at 2 dose strengths, 0.83 mcg and 1.66 mcg of desmopressin acetate per spray, for nasal administration. Each spray is 0.1 mL. The dose strengths are expressed as desmopressin acetate and are equivalent to 0.75 mcg and 1.5 mcg of desmopressin free base per spray, respectively. Both formulations also contain the following inactive ingredients: cyclopentadecanolide; cottonseed oil; sorbitan monolaurate; polysorbate 20; citric acid, anhydrous; sodium citrate dihydrate; and water for injection.
After initial priming, each actuation of NOCTIVA 0.83 mcg/0.1 mL or 1.66 mcg/0.1 mL delivers a dose of 0.83 mcg or 1.66 mcg of desmopressin acetate, respectively.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Desmopressin is a synthetic analog of vasopressin. Desmopressin is a selective agonist at V2 receptors on renal cells in the collecting ducts, increasing water reabsorption in the kidneys, and reducing urine production.
12.3 Pharmacokinetics
Absorption
Following nasal spray administration of NOCTIVA, the median time to peak plasma concentrations (Tmax) was 0.25 hour for the 0.83 mcg dose and 0.75 hour for the 1.66 mcg dose. The mean (±S.D.) peak plasma concentration (Cmax) was 4.00 (± 3.85) pg/mL for the 0.83 mcg dose and 9.11 (± 6.90) pg/mL for the 1.66 mcg dose. Plasma NOCTIVA concentrations generally declined below 2 pg/mL (lower limit of quantitation) between 4 to 6 hours post-dose.
Elimination
Following an intranasal dose of 1.66 mcg of NOCTIVA, the median apparent terminal half-life (T1/2) was 2.8 hours. The distribution of T1/2 in patients with an eGFR above 50 mL/min/1.73 m2 ranged from 1.4 to 3.8 hours.
Excretion
Desmopressin is mainly excreted in urine.
Specific Populations
Sex and Age
There were no significant differences in systemic exposure (AUC and Cmax) with respect to patient sex or age, among subjects 50 years and older.
Renal Impairment
The pharmacokinetics of NOCTIVA were evaluated in 8 renally impaired patients with an eGFR less than 50 mL/min/1.73 m2, matched to 8 patients with an eGFR greater than 50 mL/min/1.73 m2. The AUC and T1/2 of desmopressin were approximately 3- to 4-fold higher for the group with eGFR below 50 mL/min/1.73 m2. Therefore, NOCTIVA is contraindicated in patients who have renal impairment with an eGFR below 50 mL/min/1.73 m2[see Contraindications (4)].
14 CLINICAL STUDIES
The efficacy of NOCTIVA in patients with nocturia due to nocturnal polyuria was established in two 12-week randomized, double- blind, placebo-controlled, multicenter trials in adults at least 50 years of age. At baseline, patients were required to have a 6-month history of at least 2 nocturic episodes per night, on average, and at least 13 documented nocturia episodes over 6 nights during screening. The majority of patients in these trials were Caucasian (79%). The mean age was 67 years (range 50 to 90 years), 57% were men, and 43% were women.
In Trial 1, a total of 612 patients with nocturia due to nocturnal polyuria were randomized to receive either NOCTIVA 1.66 mcg (n=199), NOCTIVA 0.83 mcg (n=209), or placebo (n=204). In Trial 2, a total of 433 patients were randomized to receive NOCTIVA 1.66 mcg (n=143), 0.83 mcg (n=145), or placebo (n=145). In both trials, nocturnal polyuria was defined as a nighttime urine production exceeding one-third of the 24-hour urine production confirmed with a 24-hour urine frequency/volume chart.
Each trial had 2 co-primary efficacy endpoints: (1) The change in mean number of nocturic episodes per night from baseline during the 12-week treatment period, and (2) The percentage of patients who achieved at least a 50% reduction from baseline in the mean number of nocturia episodes per night during the 12-week treatment period.
Secondary efficacy endpoints in both trials included the percentage of nights during the treatment period with no nocturia and the percentage of nights during the treatment period with at most 1 nocturia episode. Trial 1 included a patient-reported outcome instrument known as the INTU (Impact of Nighttime Urination) questionnaire as the first-ranked secondary efficacy endpoint that assessed the impacts of nocturia on some aspects of patients’ daily lives, with an overall impact score ranging from 0 to 100 points.
Many conditions can cause nocturia. The efficacy and safety of NOCTIVA have not been established for all causes of nocturia. NOCTIVA is indicated only for patients who have nocturia due to nocturnal polyuria. The results for the co-primary efficacy endpoints among patients with nocturia due to nocturnal polyuria are shown in Table 5.
| *Intent-to-treat population: all randomized patients who received study drug and had at least 3 days of post-randomization efficacy data recorded in their diary.
CI: confidence interval †: obtained from ANCOVA model; ‡: obtained from stratified Cochran-Mantel-Haenszel (CMH) analysis. |
||||||
| Trial 1 | Trial 2 | |||||
| NOCTIVA
1.66 mcg (n=199) | NOCTIVA
0.83 mcg (n=209) | Placebo
(n=204) | NOCTIVA
1.66 mcg (n=143) | NOCTIVA
0.83 mcg (n=145) | Placebo
(n=145) |
|
| Change in Mean Number of Nocturic Episodes per Night From Baseline | ||||||
| Baseline (mean) | 3.4 | 3.4 | 3.2 | 3.3 | 3.4 | 3.4 |
| Change from
baseline† | -1.5 | -1.5 | -1.2 | -1.5 | -1.4 | -1.1 |
| Difference from
placebo† | -0.3 | -0.3 | - | -0.4 | -0.3 | - |
| 95% CI† | -0.5 to -0.1 | -0.4 to 0.0 | - | -0.6 to -0.2 | -0.5 to -0.1 | - |
| Percentage of Patients Achieving at Least a 50% Reduction in Nocturic Episodes per Night From Baseline | ||||||
| 47% | 35% | 27% | 49% | 41% | 29% | |
| Difference from
placebo‡ | 21% | 8% | - | 20% | 12% | - |
| 95% CI‡ | 12% to 30% | -0.4% to 17% | - | 9% to 31% | 1% to 23% | - |
Figure 1 shows the percentage of patients in each treatment arm who achieved various reductions from baseline in the mean number of nightly nocturic episodes. In both trials, there was consistent separation between the NOCTIVA 1.66 mcg and placebo curves.
Figure 1: Percentage of Patients Achieving Various Reductions From Baseline in Mean Number of Nightly Nocturic Episodes (Intent-to-Treat Population* With Nocturia Due to Nocturnal Polyuria)
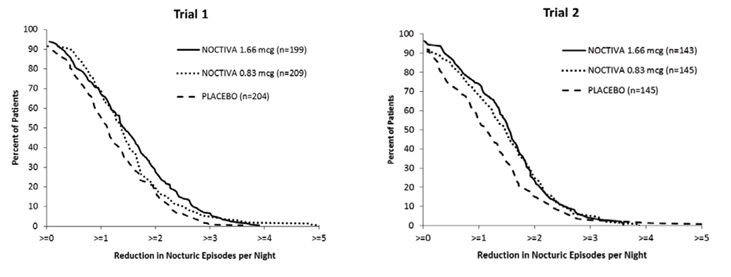
*Intent-to-treat population: all randomized patients who received study drug and had at least 3 days of post-randomization efficacy data recorded in their diary.
The results for selected secondary efficacy endpoints among patients with nocturia due to nocturnal polyuria are shown in Table 6.
| *Intent-to-treat population: all randomized patients who received study drug and had at least 3 days of post-randomization efficacy data recorded in their diary.
CI: confidence interval †: obtained from ANCOVA model. |
||||||
| Trial 1 | Trial 2 | |||||
| NOCTIVA
1.66 mcg (n=199) | NOCTIVA
0.83 mcg (n=209) | Placebo
(n=204) | NOCTIVA
1.66 mcg (n=143) | NOCTIVA
0.83 mcg (n=145) | Placebo
(n=145) |
|
| Change From Baseline in Overall Impact Score for the INTU Questionnaire (Range 0-100) | ||||||
| Baseline (mean) | 33 | 30 | 32 | |||
| Change from
baseline† | -15 | -13 | -11 | The INTU Questionnaire was not used
in Trial 2 |
||
| Difference from
placebo† | -4 | -2 | - | |||
| 95% CI† | -6 to -1 | -4 to 1 | - | |||
| Change From Baseline in Percentage of Nights With No Nocturia Episodes | ||||||
| Baseline (mean) | 0% | 0% | 0% | 0% | 0% | 0% |
| Change from
baseline† | 11% | 7% | 5% | 9% | 8% | 4% |
| Difference from
placebo† | 6% | 2% | - | 5% | 4% | - |
| 95% CI† | 2% to 10% | -1% to 6% | - | 1% to 9% | 0.4% to 8% | - |
| Change From Baseline in Percentage of Nights With at Most 1 Nocturia Episode | ||||||
| Baseline (mean) | 1% | 1% | 1% | 1% | 2% | 1% |
| Change from
baseline† | 44% | 40% | 34% | 45% | 40% | 30% |
| Difference from
placebo† | 9% | 6% | - | 15% | 10% | - |
| 95% CI† | 2% to 17% | -1% to 13% | - | 6% to 23% | 1% to 18% | - |
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
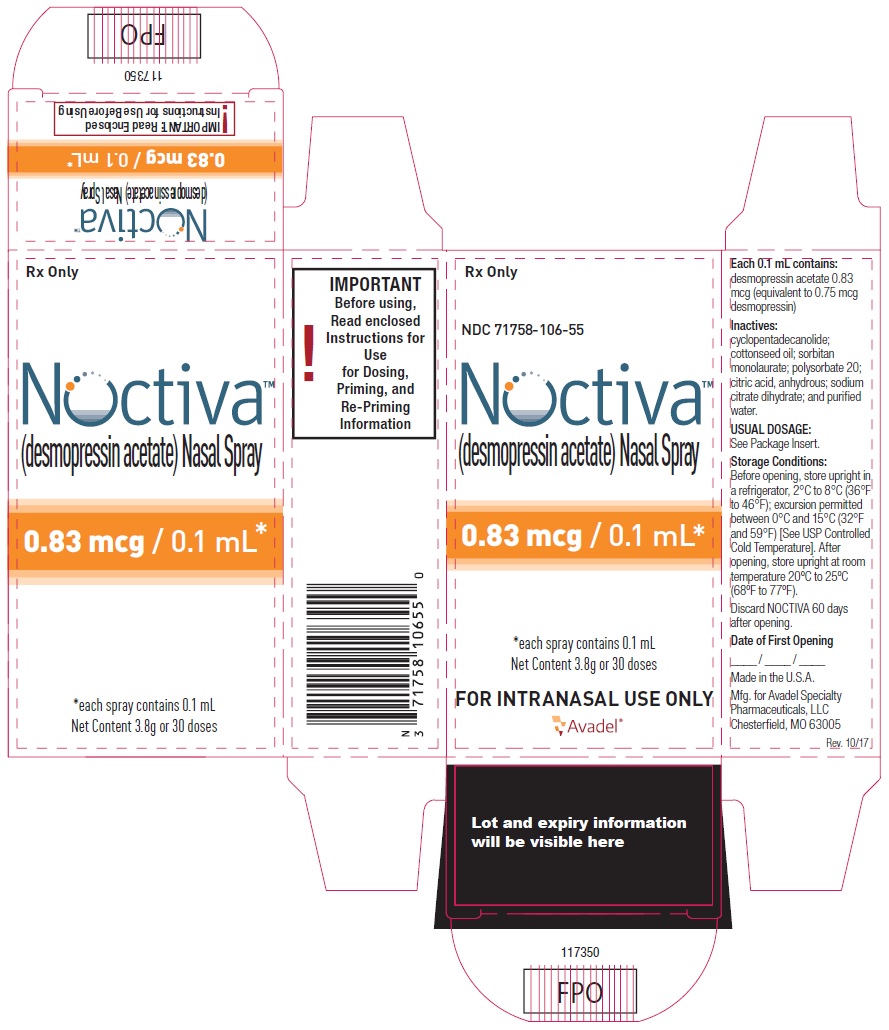
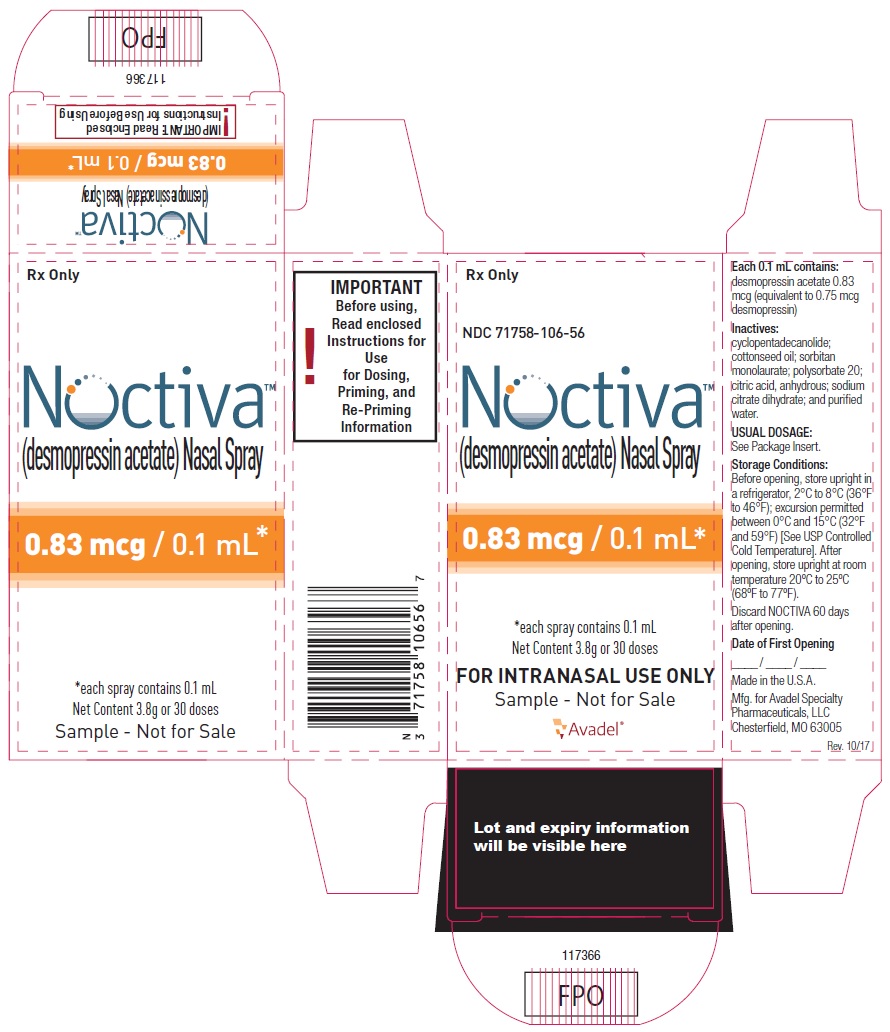
NOCTIVA (desmopressin acetate) nasal spray is available in a 3.5 mL amber glass bottle (nominal volume) fitted with a nasal actuator, a cartridge pump, and a dip tube, delivering a dose of either 0.83 mcg or 1.66 mcg of desmopressin acetate (equivalent to 0.75 mcg or 1.5 mcg of desmopressin) per spray (0.1 mL). Each bottle contains a target amount of 3.8 g formulation with 30 effective doses in addition to the initial priming (5 actuations), equivalent to 30 days of medication when used as 1 spray once a day.
| 0.83 mcg/0.1 mL of desmopressin acetate, trade bottle | NDC 71758-106-55 |
| 0.83 mcg/0.1 mL of desmopressin acetate, sample bottle | NDC 71758-106-56 |
| 1.66 mcg/0.1 mL of desmopressin acetate, trade bottle | NDC 71758-107-55 |
| 1.66 mcg/0.1 mL of desmopressin acetate, sample bottle | NDC 71758-107-56 |
16.2 Storage and Handling
- Before opening, store upright in a refrigerator, 2°C to 8°C (36°F to 46°F); excursion permitted between 0°C and 15°C (32°F and 59°F) [See USP Controlled Cold Temperature].
- After opening, store upright at room temperature 20°C to 25°C (68°F to 77°F).
- Discard NOCTIVA 60 days after opening.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Hyponatremia
Inform patients that NOCTIVA can cause hyponatremia, which may be life-threatening. Inform patients to moderate fluid intake in the evening and nighttime hours, to monitor for symptoms of hyponatremia (such as headache, nausea or vomiting, restlessness, fatigue, drowsiness, dizziness, muscle cramping, or altered mental status), to undergo recommended serum sodium measurements, to inform their health care provider about new medications, and to stop NOCTIVA during illnesses that can cause fluid or electrolyte imbalance [see Boxed Warning, Dosage and Administration (2.3), Contraindications (4), and Warnings and Precautions (5.1)].
Nasal Conditions
Inform patients to discontinue NOCTIVA if nasal conditions occur that may increase systemic absorption of NOCTIVA (e.g., atrophy of nasal mucosa, and acute or chronic rhinitis). NOCTIVA can be resumed when these conditions resolve [see Warnings and Precautions (5.3)].
Priming and Dosing
Instruct patients to prime NOCTIVA before using it for the first time by pumping 5 sprays into the air away from the face and to re-prime it by pumping 2 sprays into the air if the bottle has not been used in more than 3 days [see Dosage and Administration (2.1)].
Instruct patients not to administer 2 sprays of the 0.83 mcg dose [see Dosage and Administration (2.1)].
Manufactured for:
Avadel Specialty Pharmaceuticals, LLC
Chesterfield, MO 63005
Rev. 12/17
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Approved: 03/2017 |
|
MEDICATION GUIDE
NOCTIVA™ (nok-tee-va)
|
|
|
Important: NOCTIVA is for use in your nose (intranasal) only. |
|
|
What is the most important information I should know about NOCTIVA? NOCTIVA may cause serious side effects, including:
Call your doctor if you have any of the following symptoms of low salt levels in your blood:
Low salt levels in the blood happen more often in people who are treated with NOCTIVA and are 65 years old or older than in people treated with NOCTIVA who are younger than 65 years old. Your doctor should check the salt levels in your blood before you start taking NOCTIVA, during treatment with NOCTIVA, before increasing your dose, and before you restart taking NOCTIVA if your treatment with NOCTIVA was stopped for a period of time. To help decrease your risk of developing low levels of sodium in your blood:
Your doctor may stop your treatment with NOCTIVA for a period of time or stop your treatment completely if you have low levels of salt in your blood during treatment with NOCTIVA. |
|
|
What is NOCTIVA? NOCTIVA is a prescription medicine used in adults who wake up 2 or more times during the night to urinate due to a condition called nocturnal polyuria. Nocturnal polyuria is a condition where your body makes too much urine at night. There are other conditions that could cause you to wake up during the night to urinate. NOCTIVA is only approved for the treatment of nocturnal polyuria. Your doctor should have you measure your urine and the times that you urinate for 24 hours to determine if you have nocturnal polyuria if you have not already done this. NOCTIVA is not for use in children who wet the bed while sleeping at night. NOCTIVA has not been studied in people under 50 years of age. |
|
|
Do not use NOCTIVA if you:
Ask your doctor if you are not sure you have any of these conditions or take any of the medicines listed. |
|
|
Before using NOCTIVA, tell your doctor about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using NOCTIVA with certain other medicines may cause serious side effects. Do not start taking any new medicines until you talk to your doctor. Especially tell your doctor if you take a:
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above. Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine. |
|
|
How should I use NOCTIVA?
|
|
|
What should I avoid while taking NOCTIVA? Do not drink large amounts of fluids close to bedtime during treatment with NOCTIVA. |
|
|
What are the possible side effects of NOCTIVA? NOCTIVA can cause serious side effects.
The most common side effects of NOCTIVA include:
These are not all the possible side effects of NOCTIVA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store NOCTIVA?
Keep NOCTIVA and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of NOCTIVA. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
|
|
|
What are the ingredients in NOCTIVA? Active ingredient: desmopressin acetate Inactive ingredients: cyclopentadecanolide, cottonseed oil, sorbitan monolaurate, polysorbate 20, citric acid anhydrous, sodium citrate dihydrate, and water for injection Manufactured for Avadel Specialty Pharmaceuticals, LLC, Chesterfield, MO 63005.
|
|
Instructions for Use
NOCTIVA™ (nok-tee-va)
(desmopressin acetate) nasal spray
0.83 mcg/0.1 mL and 1.66 mcg/0.1 mL
|
Read this Instructions for Use before you start using NOCTIVA and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment. |
Important information:
- NOCTIVA is for use in your nose only.
- You will need to prime your bottle of NOCTIVA before using it for the first time. See “Before your first use, follow these instructions to prime your bottle of NOCTIVA”.
- If you do not use NOCTIVA for more than 3 days, you will need to re-prime the bottle of NOCTIVA before you start using it again. See “Re-priming NOCTIVA”.
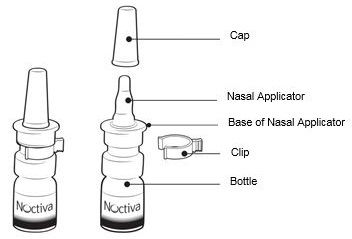
Parts of your NOCTIVA nasal spray (See Figure A).
|
Before your first use, follow these instructions to prime your bottle of NOCTIVA. |
Prime your bottle of NOCTIVA before using it for the first time.
Do not shake the bottle.
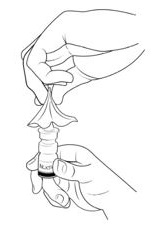
Step 1. Pull the cap off and set it aside (See Figure B).
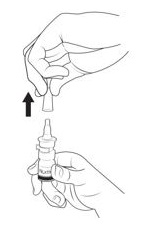
Step 2. Remove and throw away (discard) the clip (See Figure C).
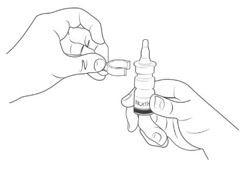
Step 3. Hold the bottle upright and away from your face.
Place 1 finger on each side of the base of the nasal applicator and your thumb underneath the bottle
(See Figure D).
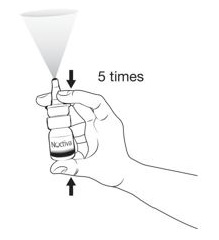
Step 4. Completely press (pump) the nasal applicator 5 times by squeezing your fingers and thumb together (See Figure E).
|
Keep nasal applicator pointed away from your face. |
Your NOCTIVA is now ready to use.
|
Using NOCTIVA |
|
Use only 1 spray of NOCTIVA in 1 nostril each night about 30 minutes before going to bed. |
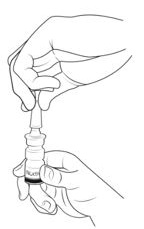
Step 1. Blow your nose to clear your nostrils (See Figure F).
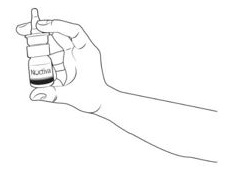
Step 2. Hold the bottle upright (See Figure G).
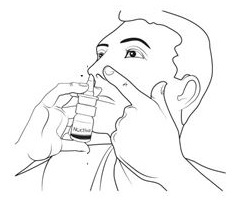
Step 3. Tilt your head back slightly (See Figure H).
Nasal applicator must be in an upright position to deliver the correct dose. Do not lie down or tilt your head too far back while giving your dose of NOCTIVA.
Step 4. Insert the nasal applicator into your left or right nostril. Keep the nasal applicator upright. Close your open nostril with a finger from your empty hand (See Figure I).
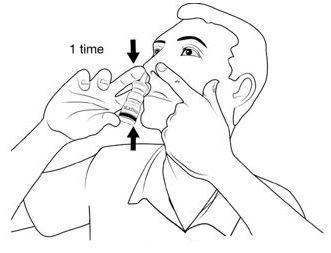
Step 5. Breathe in (inhale) gently while you pump the nasal applicator 1 time (See Figure J).
Avoid spraying in your eyes.
Gently breathe in through your nose and out through your mouth several times.
Step 6. Wipe the nasal applicator with a clean tissue (See Figure K).
Step 7. Replace the cap on the bottle (See Figure L).
|
Re-priming NOCTIVA |
If you do not use NOCTIVA for more than 3 days, you will need to re-prime the bottle before you start using it again.
If you miss a dose, take the next dose at your regular time. Do not take 2 doses at the same time.
To re-prime your bottle of NOCTIVA, hold the bottle upright and away from your face. Completely press (pump) the nasal applicator 2 times. Your NOCTIVA is now ready to use.
|
Storage:
Keep NOCTIVA and all medicines out of the reach of children. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: Avadel Specialty Pharmaceuticals, LLC
Chesterfield, MO 63005
Made in the USA
Issued: 03/2017
| NOCTIVA
desmopressin acetate spray, metered |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NOCTIVA
desmopressin acetate spray, metered |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Avadel Specialty Pharmaceuticals, LLC (080846011) |