ASPERCREME WITH LIDOCAINE- lidocaine patch
Chattem, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aspercreme Lidocaine Patch
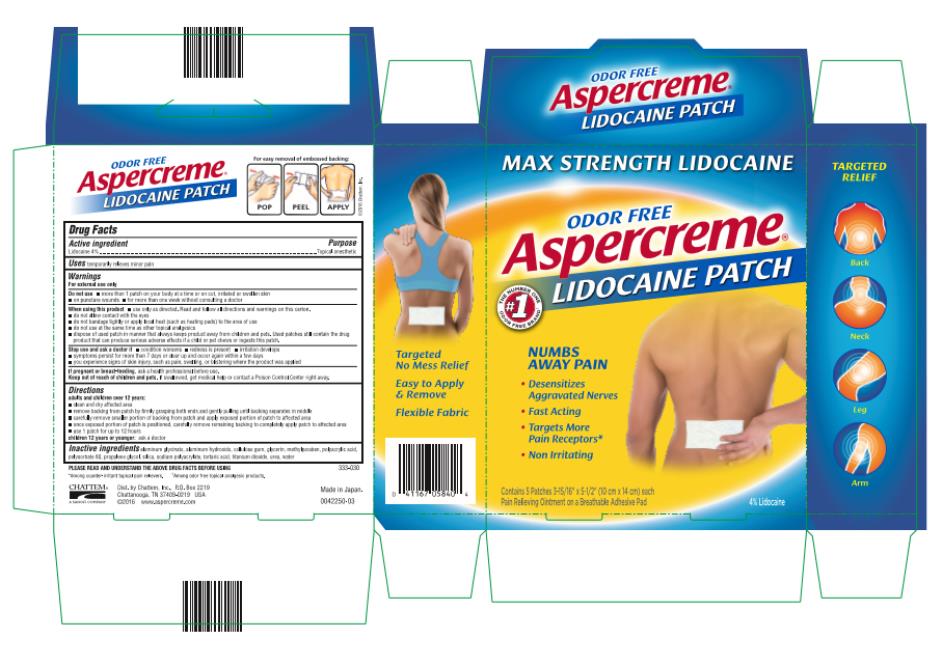
Warnings
For external use only
Do not use
- more than 1 patch on your body at a time or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- use only as directed. Read and follow all directions and warnings on this carton.
- do not allow contact with the eyes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- do not use at the same time as other topical analgesics
- dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Directions
adults and children over 12 years:
- clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- use 1 patch for up to 12 hours
children 12 years or younger: ask a doctor
| ASPERCREME WITH LIDOCAINE
lidocaine patch |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Chattem, Inc. (003336013) |
Revised: 2/2016
Document Id: 1ef99e17-b6fc-49b1-826d-2ae921f520a2
Set id: 7ad8efa9-7031-4cd0-be1d-6dee63091fd1
Version: 5
Effective Time: 20160201
Chattem, Inc.