CAFFEINE- caffeine tablet
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS 44-226-Delisted
Warnings
For occasional use only
————————————————————————————
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat.
Directions
-
adults and children 12 years and over: take 1 tablet not more often than every 3 to 4 hours
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, dextrose, dicalcium phosphate, FD&C yellow #6 aluminum lake, magnesium stearate, microcrystalline cellulose, silica gel
Principal Display Panel
CVSHealth™
Compare to the active
ingredient in Vivarin®*
NDC 59779-226-21
Caffeine Tablets
CAFFEINE, 200 mg
Alertness aid
• For mental alertness
• Safe & effective
16 TABLETS
Actual Size
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Meda Consumer Healthcare, owner of the registered trademark Vivarin®.
50844 ORG041222621
Distributed by:CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2015 CVS/pharmacy CVS.com®
1-800-SHOP CVS V-11112
CVS® Quality
Money Back Guarantee
CVS Health 44-226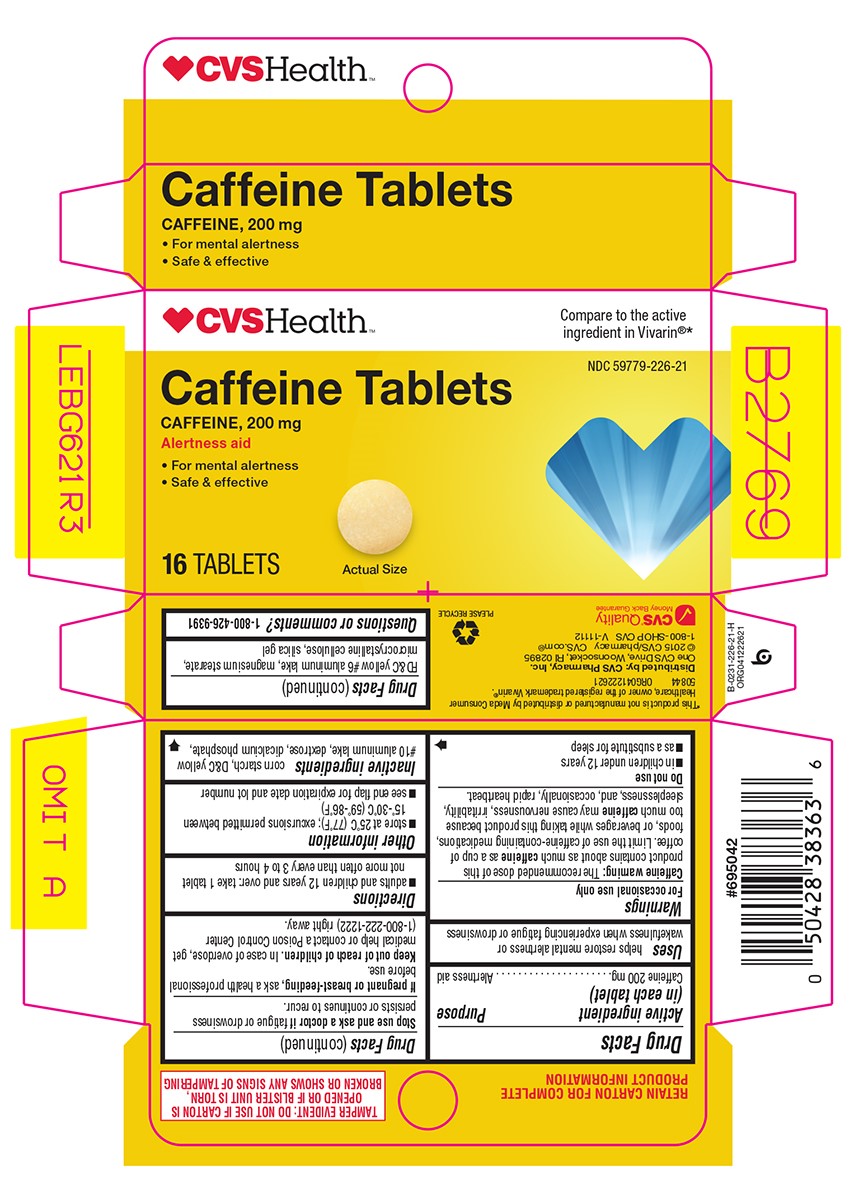
| CAFFEINE
caffeine tablet |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(59779-226) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(59779-226) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(59779-226) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(59779-226) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(59779-226) | |