INTERNATIONAL FIRST AMENITY KIT- ethyl alcohol, sodium fluoride
BUZZ PRODUCTS (HK) CO. LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
HAND CLEANSER RINSE-FREE HAND CLEANSING GEL
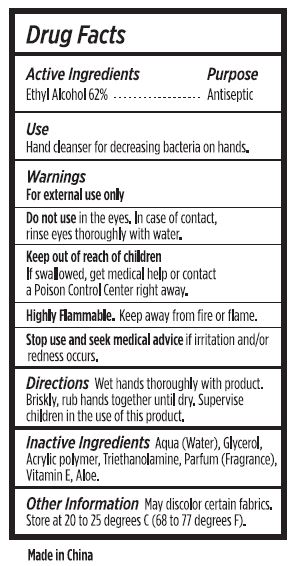
Active Ingredients
Ethyl Alcohol 62%
Use
Hand cleanser for decreasing bacteria on hands.
Warnings
For external use only
Do not use
in the eyes. In case of contact, rinse eyes thoroughly with water.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor
if irritation and/or redness develops.
Highly Flammable.
Keep away from fire or flame.
Directions
Wet hands thoroughly with product. Briskly, rub hands together until dry. Supervise children in the use of this product.
Inactive Ingredients
Aqua (Water), Glycerol, Acrylic Polymer, Triethanolamine, Parfum (Fragrance), Vitamin E, Aloe.
Other Information
May discolor certain fabrics. Store at 20 to 25 degrees C (68 to 77 degrees F).
Crest CAVITY PROTECTION Regular Paste
Active ingredient
Sodium fluoride 0.243% (0.15% w/v fluoride ion)
Purpose
Anticavity toothpaste
Use
helps protect against cavities
Keep out of reach of children under 6 yrs. of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directons
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
Inactive ingredients
sorbitol, water, hydrated silica, sodium lauryl sulfate, trisodium phosphate, flavor, sodium phosphate, cellulose gum, carbomer, sodium saccharin, titanium dioxide, blue 1
Questions?
1-800-492-7378
Package Label
PolyBag Labeling:
Bottle Labeling:

Package Labeling

BUZZ PRODUCTS (HK) CO. LIMITED


