BENZAMYCIN PAK- erythromycin and benzoyl peroxide gel
Dermik Laboratories
----------
Benzamycin® Pak
(erythromycin 3%-benzoyl peroxide 5% topical gel)
For Dermatological Use Only – Not for Ophthalmic Use
DESCRIPTION
Benzamycin® Pak contains erythromycin [(3R*,4S*,5S*,6R*,7R*,9R*,11R*,12R*,13S*,14R*)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl)-oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexa-methyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-b-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione]. Erythromycin is a macrolide antibiotic produced from a strain of Saccharopolyspora erythraea (formerlyStreptomyces erythreus). It is a base and readily forms salts with acids.
Chemically, erythromycin is (C37H67NO13). It has the following structural formula:
Erythromycin has the molecular weight of 733.94. It is a white crystalline powder and has a solubility of approximately 1 mg/mL in water and is soluble in alcohol at 25°C.
Benzamycin Pak also contains benzoyl peroxide for topical use. Benzoyl peroxide is an oxidizing agent demonstrating antibacterial activity.
Chemically, benzoyl peroxide is (C14H10O4). It has the following structural formula:
Benzoyl peroxide has the molecular weight of 242.23. It is a white granular powder and is sparingly soluble in water and alcohol and soluble in acetone, chloroform and ether.
Each gram of product, as dispensed, contains 30 mg of erythromycin and 50 mg of benzoyl peroxide in a base of SD Alcohol 40B, purified water, hydroxypropyl cellulose, Carbomer Homopolymer Type B, sodium hydroxide, dioctyl sodium sulfosuccinate 75%. Each Benzamycin Pak contains 0.8 grams of product.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Benzoyl peroxide has been shown to be absorbed by the skin where it is converted to benzoic acid. A single dose pharmacokinetic study, involving the application of either one or three units of Benzamycin Pak, was performed in 16 adult acne patients to determine systemic absorption of erythromycin. Erythromycin (with a plasma lower limit of quantitation of 2 ng/ml) was not detectable, except in one patient who was in the one unit application group.
CLINICAL STUDIES
In two adequate and well controlled clinical studies 228 patients used Benzamycin Pak, 113 patients used the currently marketed Benzamycin Topical Gel, and 183 patients used vehicle. Benzamycin Pak applied twice daily for 8 weeks was significantly more effective than vehicle and comparable to Benzamycin Topical Gel in the treatment of moderate to moderately severe facial acne vulgaris. Patients entering the study had a minimum of 15 and a maximum of 80 facial inflammatory lesions (papules and pustules) and a minimum of 20 and a maximum of 140 facial non-inflammatory lesions (open and closed comedones). The primary efficacy measures evaluated at week 8 were the lesion counts and the investigator's global assessment.
Patients were instructed to wash their face twice daily (morning and evening) with warm water and a mild cleanser provided by sponsor. No abrasive cloths or sponges, alcoholic toners, astringents or medicated solutions were used. The medication was to be applied 15 minutes after washing, in a thin film over the entire facial area. A moisturizer (supplied by the sponsor) or non-medicated make-up could be applied one hour after application, as needed. All medications were to be kept away from the eyes. Sun exposure to the face was to be limited.
Outcomes for mean percent reductions in lesion counts and investigators global assessment after 8 weeks of treatment are shown below:
| Study 1 | Benzamycin Pak
N = 119 | Benzamycin
Topical Gel N = 113 | Benzamycin Pak
Vehicle N = 38 | Benzamycin
Topical Gel Vehicle N = 37 |
|---|---|---|---|---|
|
||||
|
Mean % Lesion Counts Reduction | ||||
|
Inflammatory* |
49% |
45% |
17% |
28% |
|
Non Inflammatory* |
46% |
43% |
24% |
20% |
|
Total* |
48% |
44% |
22% |
26% |
|
Investigator's Global | ||||
|
Global Success* |
28% |
27% |
3% |
11% |
| Study 2 | Benzamycin Pak
N = 109 | Benzamycin Pak
Vehicle N = 108 |
|---|---|---|
|
||
|
Mean % Lesion Counts Reduction | ||
|
Inflammatory* |
57% |
34% |
|
Non Inflammatory |
36% |
30% |
|
Total* |
45% |
31% |
|
Investigator's Global | ||
|
Global Success* |
36% |
12% |
MICROBIOLOGY
Erythromycin acts by inhibition of protein synthesis in susceptible organisms by reversibly binding to 50 S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. Antagonism has been demonstrated in vitro between erythromycin, lincomycin, chloramphenicol and clindamycin.
Benzoyl peroxide has been shown to be effective in vitro against Propionibacterium acnes, an anaerobe found in sebaceous follicles and comedones. Benzoyl peroxide is believed to act by releasing active oxygen.
CONTRAINDICATIONS
Benzamycin Pak is contraindicated in those individuals who have shown hypersensitivity to any of its components.
PRECAUTIONS
General
For topical use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating or abrasive agents. If severe irritation develops, discontinue use and institute appropriate therapy.
The use of antibiotic agents may be associated with the overgrowth of nonsusceptible organisms. If this occurs, discontinue use and take appropriate measures.
Avoid contact with eyes and all mucous membranes.
Information for Patients
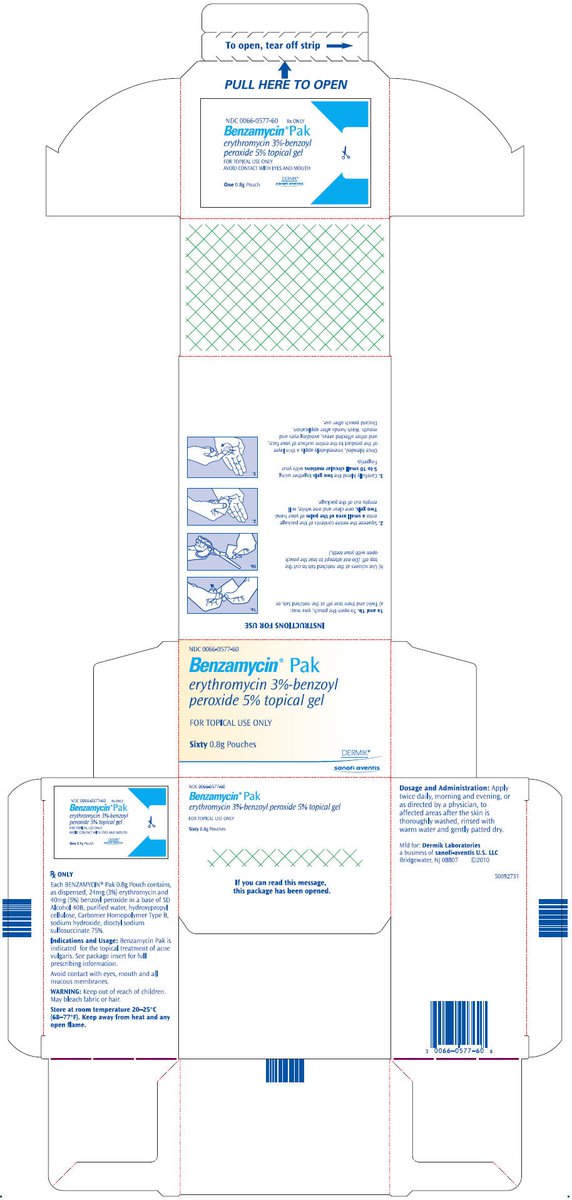
Patients using Benzamycin Pak should receive the following information and instructions:
- 1.
- Patients should be informed that they will need to mix this medication prior to use. The medication will be dispensed in one foil pouch which contains medication in two separated compartments.
- 2.
- The contents must be mixed thoroughly by the patient (in the palm of the hand), prior to application.
- 3.
- Patients should apply the product immediately after mixing, then the hands should be washed.
- 4.
- Do not mix or apply near an open flame.
- 5.
- Benzamycin Pak may bleach hair or colored fabric.
- 6.
- Excessive or prolonged exposure to sunlight should be limited. To minimize exposure to sunlight, a hat or other clothing should be worn.
- 7.
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes, mouth, and all mucous membranes as this product may be irritating.
- 8.
- Patients should report to their physician any signs of local adverse reactions.
- 9.
- This medication should not be used for any disorder other than that for which it was prescribed.
- 10.
- Patients should not use any other topical acne preparation unless otherwise directed by physician.
- 11.
- Patients should be instructed to review the instructions for use on the product carton.
- 12.
- This medication should be stored at room temperature away from heat and any open flame.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
The combination of benzoyl peroxide and erythromycin in Benzamycin Pak has not been evaluated for its carcinogenic or mutagenic potential or for its effects on fertility.
Benzoyl peroxide has been shown to be a tumor promoter and progression agent in a number of animal studies. The clinical significance of this is unknown.
Benzoyl peroxide in acetone at doses of 5 and 10 mg administered twice per week induced skin tumors in transgenic Tg.AC mice in a study using 20 weeks of topical treatment.
Benzoyl peroxide has been found to cause DNA strand breaks in a variety of mammalian cell types, to be mutagenic in Salmonella typhimurium tests by some but not all investigators, and to cause sister chromatid exchanges in Chinese hamster ovary cells.
No animal studies have been performed to evaluate the carcinogenic potential or effects on fertility of topical erythromycin. However, long-term (2-year) oral studies in rats with erythromycin base and erythromycin ethylsuccinate and in rats and mice with erythromycin stearate did not provide evidence of tumorigenicity.
The genotoxicity of erythromycin stearate has been evaluated in the Salmonella typhimurium reverse mutation assay, the mouse L5178Y lymphoma cell assay, and for sister chromatid exchanges and chromosomal aberrations in CHO cells. These studies indicated that erythromycin stearate was not genotoxic.
There was no apparent effect on male or female fertility in rats fed erythromycin base at levels up to 0.25% of diet.
Pregnancy
Teratogenic Effects: Pregnancy CATEGORY C
Animal reproduction studies have not been conducted with Benzamycin Pak or benzoyl peroxide.
There was no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base (up to 0.25% diet) prior to and during mating, during gestation and through weaning of two successive litters.
There are no well-controlled trials in pregnant women with Benzamycin Pak. It also is not known whether Benzamycin Pak can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Benzamycin Pak should be given to a pregnant woman only if clearly needed.
Nursing Women
It is not known whether the ingredients of Benzamycin Pak are excreted in human milk after topical application. However, erythromycin is excreted in human milk following oral and parenteral erythromycin administration. Therefore, caution should be exercised when erythromycin is administered to a nursing woman.
ADVERSE REACTIONS
During clinical trials, 550 acne patients were studied. Of these patients, 236 were treated with Benzamycin Pak. The most frequently reported adverse event considered at least possibly related was dry skin (7.6%) as compared to Vehicle (3.9%). Application site reactions (stinging, burning sensation, tingling, erythema) were reported in 2.5% of patients versus 1.3% for Vehicle patients. Blepharitis, pruritus and photosensitivity reactions were reported in <2% of patients who used the dual pouch product.
| Treatment Group Summaries
Number of Patients (%) |
||||
|---|---|---|---|---|
| COSTART Term | Benzamycin
Pak N = 236 | Benzamycin
Pak Vehicle N = 153 | Benzamycin
Topical Gel N = 121 | Benzamycin
Topical Gel Vehicle N = 40 |
|
DRY SKIN |
18 (7.6%) |
6 (3.9%) |
6 (5.0%) |
0 |
|
APPLICATION SITE REACTION |
6 (2.5%) |
2 (1.3%) |
1 (0.8%) |
0 |
|
BLEPHARITIS |
4 (1.7%) |
1 (0.7%) |
0 |
1 (2.5%) |
|
PRURITUS |
4 (1.7%) |
2 (1.3%) |
3 (2.5%) |
0 |
|
PHOTOSENSITIVITY REACTION |
3 (1.3%) |
0 |
0 |
0 |
|
PEELING |
1 (0.5%) |
1 (0.7%) |
0 |
0 |
DOSAGE AND ADMINISTRATION
Benzamycin Pak requires thorough mixing by the patient immediately prior to each use. The medication should be applied twice daily, morning and evening, or as directed by a physician, to affected areas after the skin is thoroughly washed, rinsed with warm water and gently patted dry.
HOW SUPPLIED
60 Pouches per carton NDC 0066-0577-60
Store at Room Temperature 20 to 25°C (68 to 77°F).
Keep away from heat and any open flame.
Keep out of the reach of children.
| BENZAMYCIN PAK
erythromycin and benzoyl peroxide gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Dermik Laboratories (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Lakewood, LLC | 077744035 | MANUFACTURE(0066-0577), ANALYSIS(0066-0577), LABEL(0066-0577), PACK(0066-0577) | |