Label: LAZANDA- fentanyl spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 71500-110-01, 71500-130-01, 71500-140-01 - Packager: West Therapeutic Development LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application
Drug Label Information
Updated March 31, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING: LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL EXPOSURE; CYTOCHROME P450 3A4 INTERACTION; CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS; RISK OF MEDICATION ERRORS; ADDICTION, ABUSE, AND MISUSE; REMS; and NEONATAL OPIOID WITHDRAWAL SYNDROME
Life-Threatening Respiratory Depression
Serious, life-threatening, and/or fatal respiratory depression has occurred in patients treated with LAZANDA, including following use in opioid non-tolerant patients and improper dosing. Monitor for respiratory depression, especially during initiation of LAZANDA or following a dose increase. The substitution of LAZANDA for any other fentanyl product may result in fatal overdose [see Warnings and Precautions ( 5.1)].
Due to the risk of respiratory depression, LAZANDA is contraindicated in the management of acute or postoperative pain including headache/migraine and in opioid non-tolerant patients. [see Contraindications ( 4)]
Accidental Exposure
Accidental exposure of even one dose of LAZANDA, especially in children, can result in a fatal overdose of fentanyl [see Warnings and Precautions ( 5.1)]. Death has been reported in children who have accidentally ingested transmucosal immediate-release fentanyl products. LAZANDA must be kept out of reach of children [see Warnings and Precautions ( 5.2)].
Cytochrome P450 3A4 Interaction
The concomitant use of LAZANDA with all cytochrome P450 3A4 inhibitors may result in an increase in fentanyl plasma concentrations, which could increase or prolong adverse reactions and may cause potentially fatal respiratory depression. In addition, discontinuation of a concomitantly used cytochrome P450 3A4 inducer may result in an increase in fentanyl plasma concentration. Monitor patients receiving LAZANDA and any CYP3A4 inhibitor or inducer [see Warnings and Precautions ( 5.3), Drug Interactions ( 7), Clinical Pharmacology ( 12.2)].
Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions ( 5.4), Drug Interactions ( 7)].
- Reserve concomitant prescribing of LAZANDA and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
Risk of Medication Errors
Substantial differences exist in the pharmacokinetic profile of LAZANDA compared to other fentanyl products that result in clinically important differences in the extent of absorption of fentanyl that could result in fatal overdose [see Dosage and Administration ( 2.1), Warnings and Precautions ( 5.5)].
- When prescribing, do not convert patients on a mcg per mcg basis from any other fentanyl products to LAZANDA.
- When dispensing, do not substitute a LAZANDA prescription for other fentanyl products.
Addiction, Abuse, and Misuse
LAZANDA exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing LAZANDA, and monitor all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions ( 5.6)].
Risk Evaluation and Mitigation Strategy (REMS) Access Program
Because of the risk for accidental exposure, misuse, abuse, addiction, and overdose, LAZANDA is available only through a restricted program required by the Food and Drug Administration, called a Risk Evaluation and Mitigation Strategy (REMS). Under the Transmucosal Immediate Release Fentanyl (TIRF) REMS, pharmacies, outpatients, and healthcare professionals who prescribe to outpatients must enroll in the program. Inpatient pharmacies must develop policies and procedures to verify opioid tolerance in inpatients who require LAZANDA while hospitalized [see Warnings and Precautions ( 5.7)] Further information is available at www.TIRFREMSaccess.com or by calling 1-866-822-1483.
Neonatal Opioid Withdrawal Syndrome
Prolonged use of LAZANDA during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Warnings and Precautions ( 5.8)]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LAZANDA safely and effectively. See full prescribing information for LAZANDA
LAZANDA ® (Fentanyl) Nasal Spray CII
Initial U.S. Approval: 1968
WARNING: LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL EXPOSURE; CYTOCHROME P450 3A4 INTERACTION; RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS; RISK OF MEDICATION ERRORS; ADDICTION, ABUSE, AND MISUSE; REMS; and NEONATAL OPIOID WITHDRAWAL SYNDROME
See full prescribing information for complete boxed warning.
- Serious, life-threatening, and/or fatal respiratory depression has occurred. Monitor closely, especially upon initiation or following a dose increase. Due to the risk of fatal respiratory depression, LAZANDA is contraindicated in opioid non-tolerant patients and in management of acute or postoperative pain, including headache/migraines. ( 4, 5.1)
- Accidental exposure of LAZANDA, especially in children, can result in a fatal overdose of fentanyl. Keep out of reach of Children. Ensure proper storage and disposal. ( 5.2)
- Concomitant use with CYP3A4 inhibitors (or discontinuation of CYP3A4 inducers) can result in a fatal overdose of fentanyl. ( 5.3, 7)
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required and follow patients for signs and symptoms of respiratory depression and sedation. ( 5.4, 7).
- When prescribing, do not convert patients on a mcg per mcg basis from any other oral transmucosal fentanyl product to LAZANDA. ( 2.1, 5.5)
- When dispensing, do not substitute with any other fentanyl products. ( 5.5)
- LAZANDA exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient’s risk before prescribing and monitor regularly for these behaviors and conditions. ( 5.6)
- LAZANDA is available only through a restricted program called the TIRF REMS. Pharmacies, outpatients, and healthcare professionals who prescribe to outpatients are required to enroll in the program. Patients must be opioid tolerant to receive a TIRF medicine. ( 5.7)
- Prolonged use of LAZANDA during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. ( 5.8)
---------------------------RECENT MAJOR CHANGES---------------------------
Boxed Warning 03/2021
Dosage and Administration (2.2) 03/2021
Warnings and Precautions ( 5.4, 5.6, 5.7) 03/2021
---------------------------INDICATIONS AND USAGE---------------------------
LAZANDA is an opiod agonist indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and who are tolerant to around-the-clock opioid therapy for their underlying persistent cancer pain. ( 1)
Patients considered opioid tolerant are those who are taking, for one week or longer, around-the-clock medicine consisting of at least 60 mg of oral morphine per day, at least 25 mcg of transdermal fentanyl per hour, at least 30 mg of oral oxycodone per day, at least 8 mg of oral hydromorphone per day, at least 25 mg oral oxymorphone per day, at least 60 mg oral hydrocodone per day, or an equianalgesic dose of another opioid daily for a week or longer. Patients must remain on around-the-clock opioids while taking LAZANDA.
Limitations of Use
- Not for use in opioid non-tolerant patients.
- Not for use in the management of acute or postoperative pain, including headache/migraine, dental pain, or in the emergency room.
- As a part of the TIRF REMS, LAZANDA may be dispensed by outpatient pharmacies only to outpatients enrolled in the program (5.7). For inpatient administration of LAZANDA, patient and prescriber enrollment are not required.
-----------------------DOSAGE AND ADMINISTRATION-----------------------
- Patients must require and use around-the-clock opioids when taking LAZANDA. ( 1)
- Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals ( 2.1)
- Individualize dosing based on the severity of pain, patient response, prior analgesic experience, and risk factors for addiction, abuse, and misuse. ( 2.1)
- Initial dose of LAZANDA for all patients is 100 mcg (single spray into one nostril. (2.3)
- Individually titrate to an effective dose, from 100 mcg to 200 mcg to 300 mcg to 400 mcg to 600 mcg, and up to a maximum of 800 mcg, that provides adequate analgesia with tolerable side effects. (2.4)
- Dose is a single spray into one nostril, a single spray into each nostril (2 sprays), three single sprays (alternating nostrils), or two sprays into each nostril (4 sprays); no more than four doses per 24 hours. (2.3, 2.4)
- Wait at least 2 hours before treating another episode of breakthrough pain with LAZANDA. (2.3)
- During any episode, if adequate pain relief is not achieved within 30 minutes, the patient may use a rescue medication as directed by their healthcare provider. (2.4)
- When opioid therapy is no longer required, consider discontinuing LAZANDA along with a gradual downward of other opioids to minimize possible withdrawal effects. (2.7)
- Discuss availability of naloxone with the patient and caregiver and assess each patient’s need for access to naloxone, both when initiating and renewing treatment with LAZANDA. Consider prescribing naloxone based on the patient’s risk factors for overdose [2.2, 5.1, 5.4, 5.6]
-----------------------DOSAGE FORMS AND STRENGTHS-----------------------
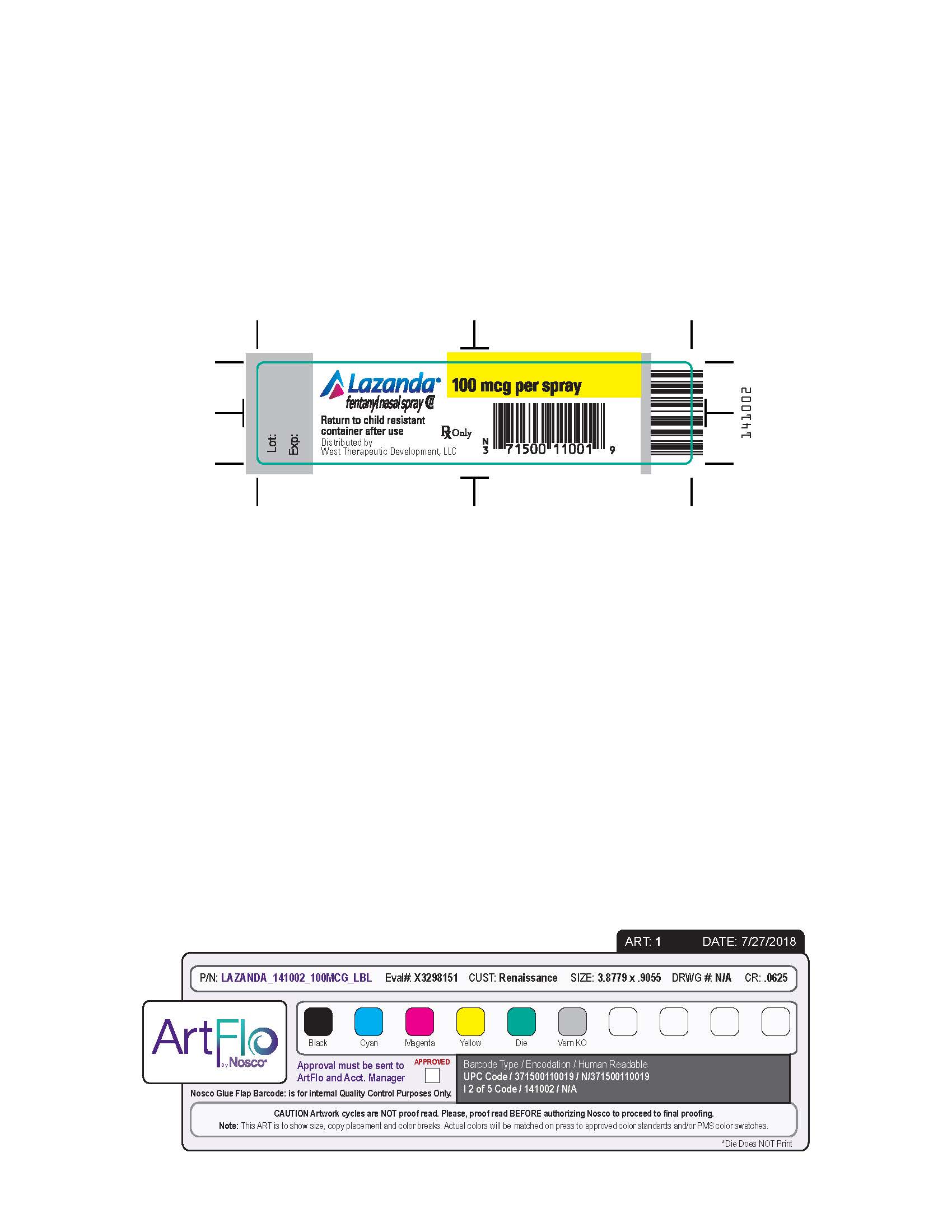
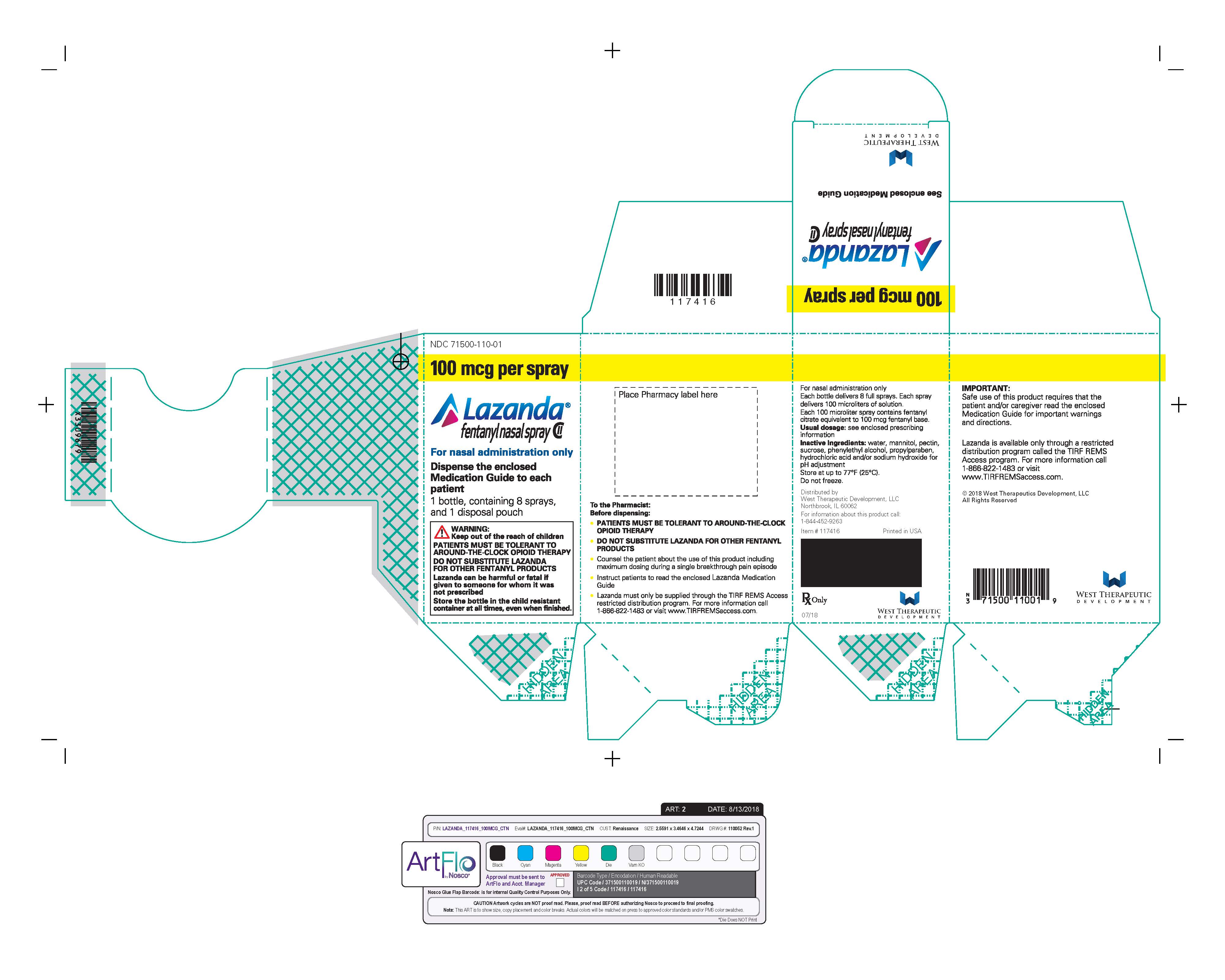
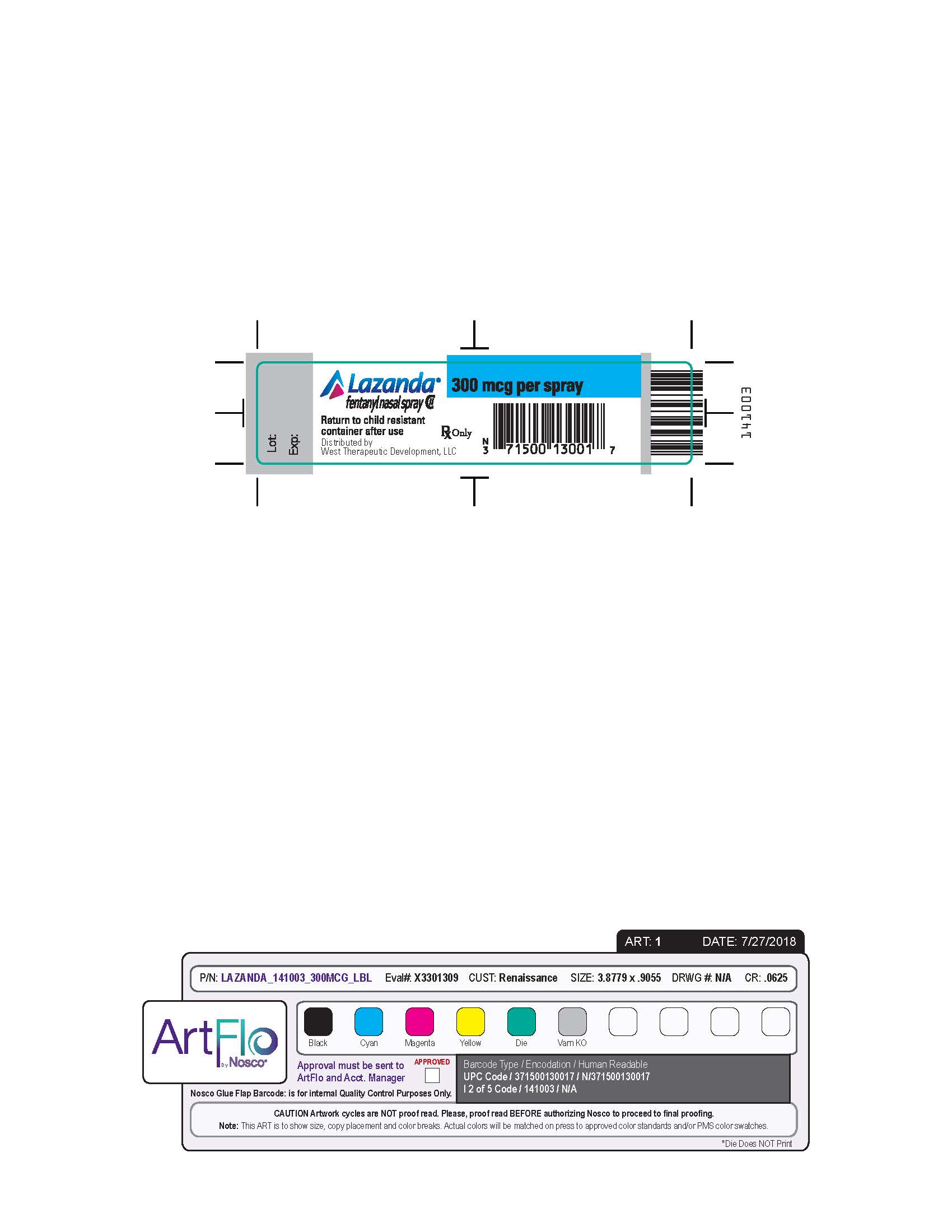
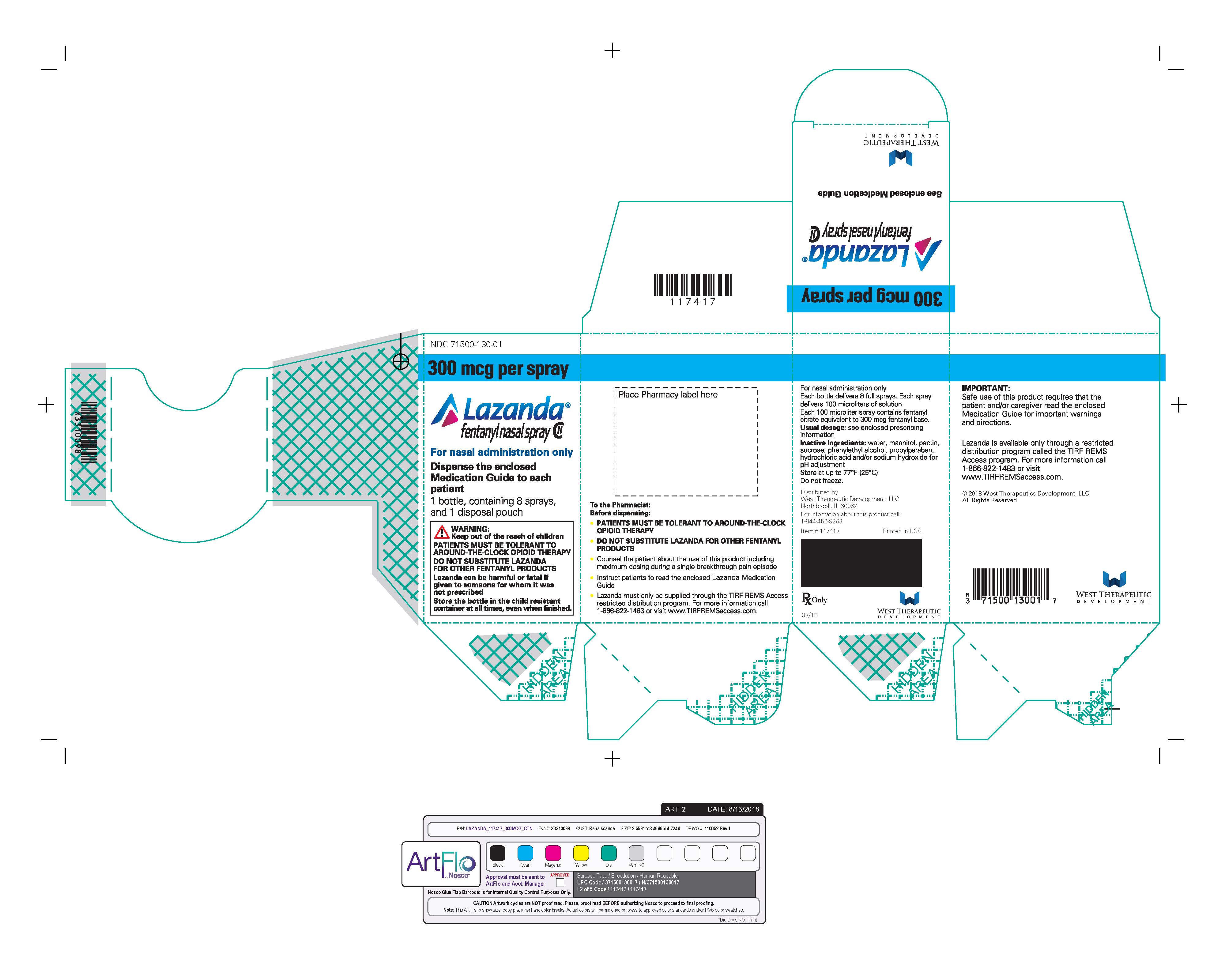
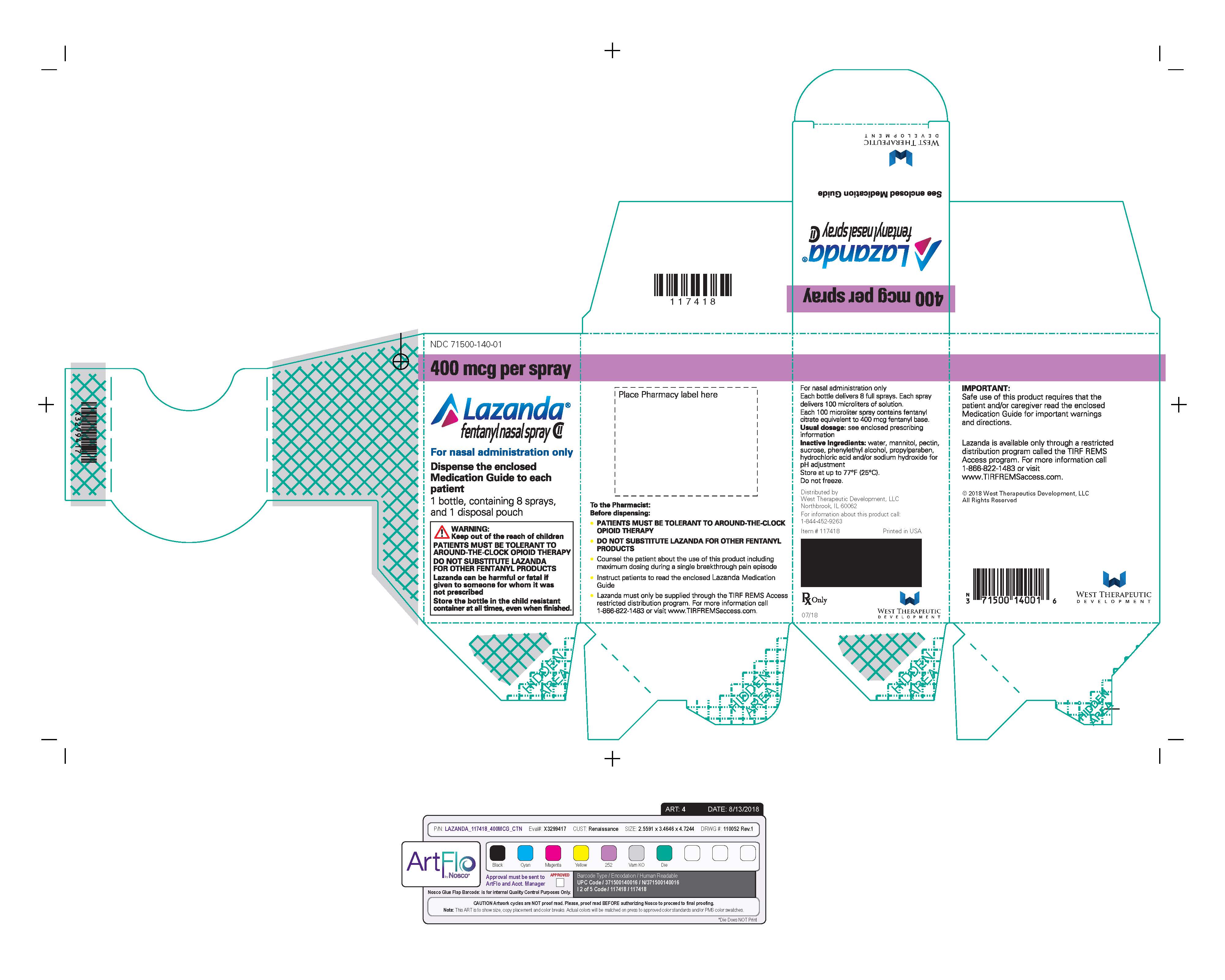
- Nasal spray: each spray delivers 100 mcL of solution containing either 100 mcg, 300 mcg or 400 mcg fentanyl base. ( 3) Supplied in a 5 mL bottle containing 8 sprays.
-----------------------------------CONTRAINDICATIONS-------------------------
- Opioid non-tolerant patients ( 4)
- Management of acute or postoperative pain including headache/migraine and dental pain, or in emergency department. ( 4)
- Significant respiratory depression. ( 4)
- Acute or severe bronchial asthma in an unmonitored setting or in absence of resuscitative equipment. ( 4)
- Known or suspected gastrointestinal obstruction, including paralytic ileus. ( 4)
- Known hypersensitivity to fentanyl or components of LAZANDA. ( 4)
---------------------------WARNINGS AND PRECAUTIONS--------------------
- Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration. ( 5.9)
- Serotonin Syndrome: Potentially life-threatening condition could result from concomitant serotonergic drug administration. Discontinue LAZANDA if serotonin syndrome is suspected. ( 5.10)
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. ( 5.11)
- Severe Hypotension: Monitor during dosage initiation and titration. Avoid use of LAZANDA in patients with circulatory shock. ( 5.12)
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. Avoid use of LAZANDA in patients with impaired consciousness or coma. ( 5.13)
----------------------------------ADVERSE REACTIONS---------------------------
- Most common adverse reactions (incidence ≥5%) were vomiting, nausea, dizziness, pyrexia, and constipation. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact West Therapeutic Development, LLC at 1-844-452-9263 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
--------------------------------DRUG INTERACTIONS----------------------------
- Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: Avoid use with LAZANDA because they may reduce analgesic effect of LAZANDA or precipitate withdrawal symptoms. ( 7)
---------------------------USE IN SPECIFIC POPULATIONS---------------------
- Pregnancy: May cause fetal harm. ( 8.1)
- Lactation: Not Recommended. ( 8.2)
- Renal and Hepatic Impairment: Administer LAZANDA with caution. ( 8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 03/2021
- FULL PRESCRIBING INFORMATION: CONTENTS
-
INDICATIONS AND USAGE
LAZANDA is indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and who are tolerant to around-the-clock opioid therapy for their underlying persistent cancer pain.
Patients considered opioid tolerant are those who are taking, for one week or longer, around-the-clock medicine consisting of at least: 60 mg of oral morphine per day, 25 mcg of transdermal fentanyl per hour, 30 mg oral oxycodone per day, 8 mg oral hydromorphone per day, or at least 25 mg oral oxymorphone per day, or at least 60mg oral hydrocodone per day, or an equianalgesic dose of another opioid for a week or longer. Patients must remain on around-the-clock opioids when taking LAZANDA.
Limitations of Use:
- Not for use in opioid non-tolerant patients.
- Not for use in the management of acute or postoperative pain, including headache/migraine, dental pain, or in the emergency department [see Contraindications (4)].
- As a part of the TIRF REMS, LAZANDA may be dispensed by outpatient pharmacies only to outpatients enrolled in the program. [see Warnings and Precautions (5.7)]. For inpatient administration of LAZANDA, patient and prescriber enrollment are not required.
-
DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Healthcare professionals who prescribe LAZANDA for outpatients must enroll in the TIRF REMS and comply with the requirements of the REMS to ensure safe use of
LAZANDA [see Warnings and Precautions ( 5.7)].
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals.
- It is important to minimize the number of strengths available to patients at any time to prevent confusion and possible overdose.
- Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse.
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with LAZANDA and adjust the dosage accordingly [see Warnings and Precautions ( 5.1)].
- Instruct patients and caregivers to take steps to store LAZANDA securely and to properly dispose of unused LAZANDA as soon as no longer needed [see Warnings and Precautions ( 5.2, 5.6), Patient Counseling Information ( 17)].
- LAZANDA is not bioequivalent with other fentanyl products. Do not convert patients on a mcg per mcg basis from other fentanyl products. There are no conversion directions available for patients on any other fentanyl products (Note: This includes oral, transdermal, or parenteral formulations of fentanyl) [see Warnings and Precautions ( 5.5)].
- LAZANDA is NOT a generic version of any other oral transmucosal fentanyl product [see Warnings and Precautions ( 5.5)].
2.2 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver and assess the potential need for access to naloxone, both when initiating and renewing treatment with LAZANDA [see Warnings and Precautions (5.1), Patient Counseling Information ( 17)].
Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Consider prescribing naloxone, based on the patient’s risk factors for overdose, such as concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose. The presence of risk factors for overdose should not prevent the proper management of pain in any given patient [see Warnings and Precautions ( 5.1, 5.4, 5.6)].
Consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or overdose.
2.3 Initial Dosage
Initiate treatment with LAZANDA for all patients (including those switching from another fentanyl product) using ONE 100 mcg spray of LAZANDA (1 spray in one nostril).
Repeat Dosing
- If adequate analgesia is obtained within 30 minutes of administration of the 100 mcg single spray, treat subsequent episodes of breakthrough pain with this dose.
- If adequate analgesia is not achieved with the first 100 mcg dose, dose escalate in a step-wise manner over consecutive episodes of breakthrough pain until adequate analgesia with tolerable side effects is achieved.
- Patients MUST wait at least 2 hours before treating another episode of breakthrough cancer pain with LAZANDA.
2.4 Titration and Maintenance of Therapy
Titration
The objective of dose titration is to identify an effective and tolerable maintenance dose for ongoing management of breakthrough cancer pain episodes. The effective and tolerable dose of LAZANDA will be determined by dose titration in individual patients.
Titration steps: If adequate analgesia is not achieved with the first 100 mcg dose, dose escalate in a step-wise manner over consecutive episodes of breakthrough pain until adequate analgesia with tolerable side effects is achieved.
Patients MUST wait at least 2 hours before treating another episode of breakthrough cancer pain with LAZANDA.
The titration steps should be:
LAZANDA Dose How to administer the dose 100 mcg Using the 100 mcg dose; one spray in one nostril 200 mcg Using the 100 mcg dose; a total of two sprays, as one spray in each nostril 300 mcg Using the 100 mcg dose; a total of three sprays, alternating one spray in right nostril, second spray in left nostril, third spray in right nostril 400 mcg Using the 100 mcg dose, a total of four sprays, alternating one spray in right nostril, second spray in left nostril, third spray in right nostril, and fourth spray in left nostril
OR
Using the 400 mcg dose; one spray in one nostril600 mcg Using the 300 mcg dose; total of two sprays, as one spray in each nostril 800 mcg Using the 400 mcg dose; total of two sprays, as one spray in each nostril Patients should confirm the dose of LAZANDA that works for them with a second episode of breakthrough pain and review their experience with their physicians to determine if that dose is appropriate, or whether a further adjustment is warranted.
The safety and efficacy of doses higher than 800 mcg have not been evaluated in clinical studies. Avoid the use of a combination of dose strengths to treat an episode as this may cause confusion and dosing errors.
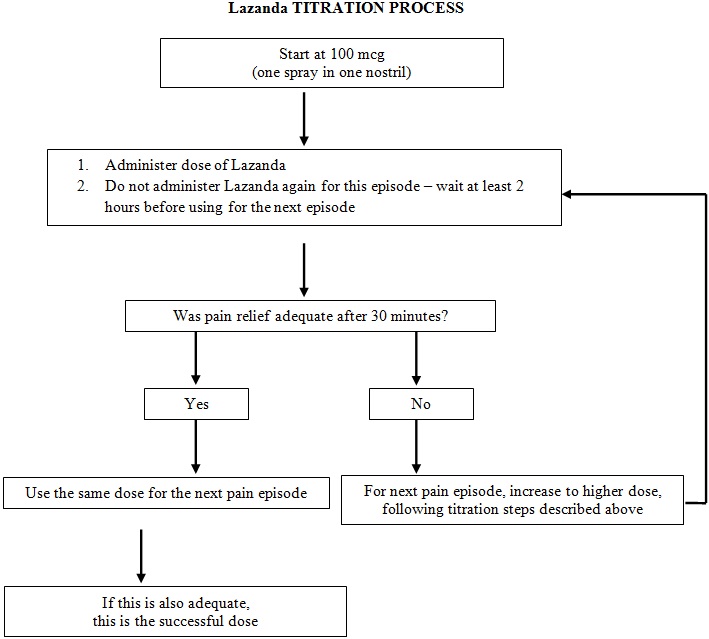
In order to minimize the risk of LAZANDA-related adverse reactions and to identify the appropriate dose, it is imperative that patients be supervised closely by health professionals during the titration process.
Maintenance Therapy
Once an appropriate dose has been established, instruct patients to use that dose for each subsequent breakthrough cancer pain episode. Limit LAZANDA use to four or fewer doses per day.
Patients MUST wait at least 2 hours before treating another episode of breakthrough cancer pain with LAZANDA.
During any episode of breakthrough cancer pain, if there is inadequate pain relief after 30 minutes following LAZANDA dosing or if a separate episode of breakthrough cancer pain occurs before the next dose of LAZANDA is permitted (i.e., within 2 hours), the patients may use a rescue medication as directed by their healthcare provider.
2.5 Dose Re-Adjustment
If the response (analgesia or adverse reactions) to the titrated LAZANDA dose markedly changes, an adjustment of dose may be necessary to ensure that an appropriate dose is maintained.
If more than four episodes of breakthrough pain are experienced per day, re-evaluate the dose of the long-acting opioid used for persistent underlying cancer pain. If the long-acting opioid or dose of long-acting opioid is changed, re-evaluate and re-titrate the LAZANDA dose as necessary to ensure the patient is on an appropriate dose.
Limit the use of LAZANDA to treat four or fewer episodes of breakthrough pain per day.
It is imperative that any dose re-titration is monitored carefully by a healthcare professional.
2.6 Administration of LAZANDA
Instruct patients on the proper use of LAZANDA.
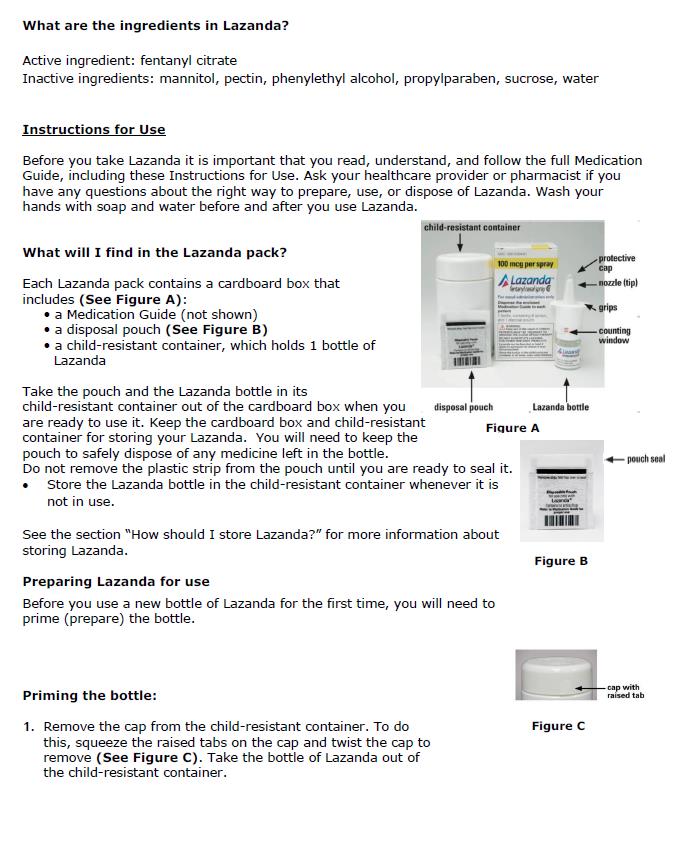
- Prime the device before use by spraying into the pouch (4 sprays in total). If the product has not been used for 5 days, re-prime by spraying once. For priming, follow the instructions provided [See Medication Guide].
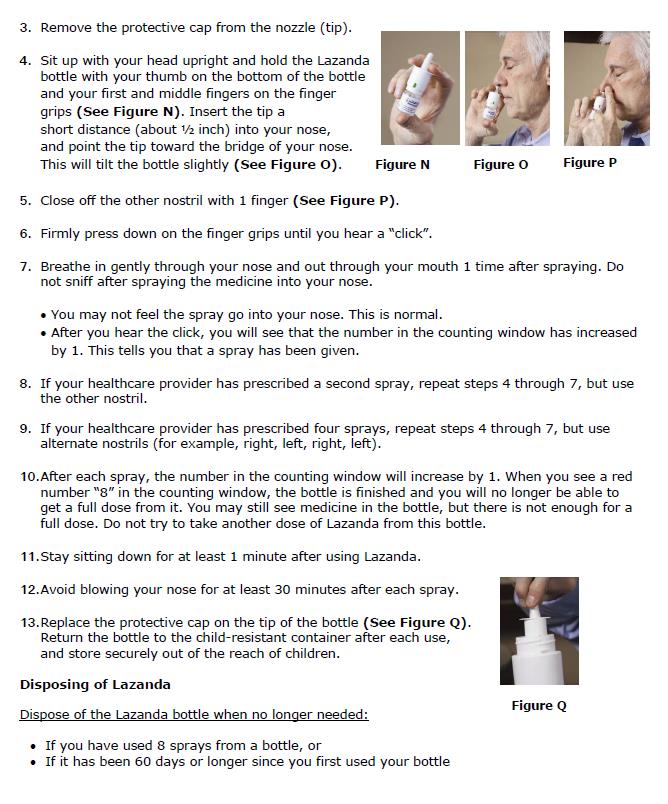
- Insert the nozzle of the LAZANDA bottle a short distance (about ½ inch or 1 cm) into the nose and point towards the bridge of the nose, tilting the bottle slightly.
- Press down firmly on the finger grips until they hear a “click” and the number in the counting window advances by one.
Advise patients that the fine mist spray is not always felt on the nasal mucosal membrane and to rely on the audible click and the advancement of the dose counter to confirm a spray has been administered.
2.7 Discontinuation of Therapy
For patients no longer requiring opioid therapy, consider discontinuing LAZANDA along with a gradual downward titration of other opioids to minimize possible withdrawal effects. In patients who continue to take their chronic opioid therapy for persistent pain but no longer require treatment for breakthrough pain, LAZANDA therapy can usually be discontinued immediately [see Drug Abuse and Dependence ( 9.3)].
2.8 Disposal of LAZANDA
Instruct patients and caregivers to properly dispose of all unused, partially used and used LAZANDA bottles. The remaining liquid in all bottles must be sprayed into the pouch, provided in the pack, for safe disposal as soon as possible.
Instruct the patient how to do this correctly. If there are any unwanted therapeutic sprays remaining in the bottle, instruct the patient to spray these into the pouch until the number “8” appears in the counting window and there are no more full therapeutic sprays obtainable from the bottle. After the counter has advanced to “8”, the patient should continue to push down on the finger grips a total of four times in order to expel any residual medicine from the bottle. After the 8 therapeutic sprays have been emitted, the patient will not hear a click and the counter will not advance beyond “8”; further sprays emitted will not be full sprays and should always be trapped in the pouch, not used therapeutically.
Instruct the patient and caregiver to seal the pouch and place both it and the empty bottle into the child-resistant storage container. Patients must wash their hands with soap and water immediately after handling the pouch.
The patient must discard the child-resistant container containing the pouch and the bottle in the trash.
The patient or caregiver must continue to store the LAZANDA bottle in the specially provided child-resistant container and the pouch out of the reach of children until proper disposal, as described above, is possible.
Instruct the patient to dispose of the LAZANDA bottle and start a new one if it has been 60 days or more since they first used the bottle of LAZANDA.
In the event that caregivers or patients require additional assistance with the disposal of LAZANDA bottles, call the West Therapeutic Development, LLC toll-free number (1-844-452-9263).
- DOSAGE FORMS AND STRENGTHS
-
CONTRAINDICATIONS
LAZANDA is contraindicated in:
· Opioid non-tolerant patients: Life-threatening respiratory depression and death could occur at any dose in opioid non-tolerant patients [see Indications and Usage (1); Warnings and Precautions (5.1)].
· Acute or postoperative pain including headache/migraine and dental pain, or in the emergency department.
· Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see Warnings and Precautions (5.9)]
· Known or suspected gastrointestinal obstruction, including paralytic ileus [see Warnings and Precautions (5.14)]
Known hypersensitivity to fentanyl or components of LAZANDA (e.g., anaphylaxis, hypersensitivity) [see Adverse Reactions (6.2)].
-
WARNINGS AND PRECAUTIONS
5.1 Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status [see Overdosage ( 10)]. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of LAZANDA, the risk is greatest during the initiation of therapy or following a dosage increase. Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy with and following dosage increases of LAZANDA.
To reduce the risk of respiratory depression, proper dosing and titration of LAZANDA are essential [see Dosage and Administration ( 2.1)]. Overestimating the LAZANDA dosage can result in a fatal overdose with the first dose. The substitution of LAZANDA for any other fentanyl product may result in fatal overdose [see Warnings and Precautions ( 5.5)].
LAZANDA could be fatal to individuals for whom it is not prescribed and for those who are not opioid-tolerant.
Accidental ingestion of (or exposure to) even one dose of LAZANDA, especially by (in) children, can result in respiratory depression and death due to an overdose of fentanyl [see Warnings and Precautions ( 5.2)].
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Patient Counseling Information ( 17)].
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [ see Dosage and Administration (2.5)].
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver and assess the potential need for access to naloxone, both when initiating and renewing treatment with LAZANDA. Inform patients and caregivers about the various ways to obtain naloxone as permitted
by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program). Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help, even if naloxone is administered [see Patient Counseling Information ( 17)].
Consider prescribing naloxone, based on the patient’s risk factors for overdose, such as concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose. The presence of risk factors for overdose should not prevent the proper management of pain in any given patient. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or overdose. If naloxone is prescribed, educate patients and caregivers on how to treat with naloxone. [see Warnings and Precautions ( 5.4, 5.6), Patient Counseling Information ( 17)].
5.2 Increased Risk of Overdose in Children Due to Accidental Ingestion or Exposure
Death has been reported in children who have accidentally ingested transmucosal immediate –release fentanyl products.
Patients and their caregivers must be informed that LAZANDA contains a medicine in an amount which can be fatal to a child. Physicians and dispensing pharmacists must specifically question patients or caregivers about the presence of children in the home (on a full time or visiting basis) and counsel them regarding the dangers to children from inadvertent exposure.
Patients and their caregivers must be instructed to keep both used and unused dosage units out of the reach of children. While all units should be disposed of immediately after use, partially consumed units represent a special risk to children. In the event that a unit is not completely consumed it must be properly disposed as soon as possible [see Patient Counseling Information ( 17)].
Detailed instructions for the proper storage, administration, disposal, and important instructions for managing an overdose of LAZANDA are provided in the LAZANDA Medication Guide. Encourage patients to read this information in its entirety and give them an opportunity to have their questions answered.
5.3 Risks of Concomitant Use or Discontinuation of Cytochrome P450 3A4 Inhibitors and Inducers
Concomitant use of LAZANDA with a CYP3A4 inhibitor, such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritonavir), may increase plasma concentrations of fentanyl and prolong opioid adverse reactions, which may cause potentially fatal respiratory depression [see Warnings and Precautions (5.2)], particularly when an inhibitor is added after a stable dose of LAZANDA is achieved. Similarly, discontinuation of a CYP3A4 inducer, such as rifampin, carbamazepine, and phenytoin, in LAZANDA treated patients may increase fentanyl plasma concentrations and prolong opioid adverse reactions. When using LAZANDA with CYP3A4 inhibitors or discontinuing CYP3A4 inducers in LAZANDA treated patients, monitor patients closely at frequent intervals and consider dosage reduction of LAZANDA until stable drug effects are achieved [see Dosage and Administration (2.4), Drug Interactions (7)].
Concomitant use of LAZANDA with CYP3A4 inducers or discontinuation of an CYP3A4 inhibitor could decrease fentanyl plasma concentrations, decrease opioid efficacy or, possibly, lead to a withdrawal syndrome in a patient who had developed physical dependence to fentanyl. When using LAZANDA with CYP3A4 inducers or discontinuing CYP3A4 inhibitors, monitor patients closely at frequent intervals and consider increasing the opioid dosage if needed to maintain adequate analgesia or if symptoms of opioid withdrawal occur [see Drug Interactions (7)].
5.4 Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Profound sedation, respiratory depression, coma, and death may result from the concomitant use of LAZANDA with benzodiazepines or other CNS depressants (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see Drug Interactions ( 7)].
If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Follow patients closely for signs and symptoms of respiratory depression and sedation.
If concomitant use is warranted, consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration ( 2.2), Warnings and Precautions ( 5.1)].
Advise both patients and caregivers about the risks of respiratory depression and sedation when LAZANDA is used with benzodiazepines or other CNS depressants (including alcohol and illicit drugs). Advise patients not to drive or operate heavy machinery until the effects of concomitant use of the benzodiazepine or other CNS depressant have been determined. Screen patients for risk of substance use disorders, including opioid abuse and misuse, and warn them of the risk for overdose and death associated with the use of additional CNS depressants including alcohol and illicit drugs [see Drug Interactions ( 7), Patient Counseling Information ( 17)].
5.5 Risk of Medication Errors
When prescribing, DO NOT convert a patient to LAZANDA from any other fentanyl product on a mcg per mcg basis as LAZANDA and other fentanyl products are not equivalent on a microgram per microgram basis.
LAZANDA is NOT a generic version of other transmucosal immediate release fentanyl (TIRF) formulations. When dispensing, DO NOT substitute a LAZANDA prescription for any other TIRF formulation under any circumstances. Other TIRF formulations and LAZANDA are not equivalent. Substantial differences exist in the pharmacokinetic profile of LAZANDA compared to other fentanyl products including other TIRF formulations that result in clinically important differences in the rate and extent of absorption of fentanyl. As a result of these differences, the substitution of LAZANDA for any other fentanyl product may result in a fatal overdose.
There are no safe conversion directions available for patients on any other fentanyl products. (Note: This includes oral, transdermal, or parenteral formulations of fentanyl.) Therefore, for opioid tolerant patients, the initial dose of LAZANDA should always be ONE 100 mcg spray. Each patient should be individually titrated to provide adequate analgesia while minimizing side effects [see Dosage and Administration ( 2.4)].
5.6 Addiction, Abuse, and Misuse
LAZANDA contains fentanyl a Schedule II controlled substance. As an opioid, LAZANDA exposes users to the risks of addiction, abuse, and misuse [see Drug Abuse and Dependence (9)].
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed LAZANDA. Addiction can occur at recommended dosages and if the drug is misused or abused.
Assess each patient’s risk for opioid addiction, abuse, or misuse prior to prescribing LAZANDA, and monitor all patients receiving LAZANDA for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as LAZANDA, but use in such patients necessitates intensive counseling about the risks and proper use of LAZANDA along with intensive monitoring for signs of addiction, abuse, and misuse. Consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration ( 2.2), Warnings and Precautions ( 5.1)].
Opioids are sought by drug abusers and people with addiction disorders and are subject to criminal diversion. Consider these risks when prescribing or dispensing LAZANDA. Strategies to reduce these risks include prescribing the drug in the smallest appropriate quantity and advising the patient on the proper disposal of unused drug [see Patient Counseling Information (17)]. Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
5.7 Transmucosal Immediate Release Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS)
Because of the risk for accidental exposure, misuse, abuse, addiction, and overdose [see Warnings and Precautions (5.6)], LAZANDA is available only through a restricted program under a REMS called the TIRF REMS. Under the TIRF REMS, healthcare professionals who prescribe to outpatients, the outpatients themselves, and pharmacies are required to enroll in the program.
Notable requirements of the TIRF REMS are:
- Prescribers for outpatient use must must be certified by the TIRF REMS program by enrolling and completing training. Prescribers must document opioid tolerance with every LAZANDA prescription.
- Outpatients must be enrolled in the REMS program, and must be opiod tolerant to receive LAZANDA [see Dosage and Administration ( 2.1)]
- Outpatient pharmacies must be certified with the REMS program and verify documentation of opioid tolerance with every LAZANDA prescription.
- Inpatient pharmacies must be certified with the REMS program and develop policies and procedures to verify opioid tolerance in inpatients who require LAZANDA while hospitalized.
- Wholesalers and distributers must enroll in the REMS program and distribute only to certified pharmacies.
- Further information, including a list of certified pharmacies and enrolled distributors, is available at www.tirfremsaccess.com or by calling 1-866-822-1483.
5.8 Neonatal Opioid Withdrawal Syndrome
Prolonged use of LAZANDA during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for a prolonged period of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Use in Specific Populations ( 8.1), Patient Counseling Information ( 17)].
5.9 Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
The use of LAZANDA in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease: LAZANDA-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive including apnea, even at recommended dosages of LAZANDA [see Warnings and Precautions (5.1)].
Elderly, Cachectic, or Debilitated Patients: Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients because they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients [see Warnings and Precautions (5.1)].
Monitor such patients closely, particularly when initiating and titrating LAZANDA and when LAZANDA is given concomitantly with other drugs that depress respiration [see Warnings and Precautions (5.1)]. Alternatively, consider the use of non-opioid analgesics in these patients.
5.10 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of fentanyl with serotonergic drugs. Serotonergic drugs include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonergic neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), and drugs that impair metabolism of serotonin (including MAO inhibitors, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue) [see Drug Interactions (7)]. This may occur within the recommended dosage range.
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms generally occurs within several hours to a few days of concomitant use, but may occur later than that. Discontinue LAZANDA if serotonin syndrome is suspected.
5.11 Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
5.12 Severe Hypotension
LAZANDA may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g. phenothiazines or general anesthetics) [see Drug Interactions ( 7)]. Monitor these patients for signs of hypotension after initiating or titrating the dosage of LAZANDA. In patients with circulatory shock, LAZANDA may cause vasodilation that can further reduce cardiac output and blood pressure. Avoid the use of LAZANDA in patients with circulatory shock.
5.13 Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
In patients who may be susceptible to the intracranial effects of CO 2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), LAZANDA may reduce respiratory drive, and the resultant CO 2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with LAZANDA.
Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of LAZANDA in patients with impaired consciousness or coma.
5.14 Risks of Use in Patients with Gastrointestinal Conditions
LAZANDA is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The fentanyl in LAZANDA may cause spasm of the sphincter of Oddi. Opioids may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis for worsening symptoms.
5.15 Increased Risk of Seizures in Patients with Seizure Disorders
The fentanyl in LAZANDA may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures occurring in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during LAZANDA therapy.
5.16 Risks of Driving and Operating Machinery
LAZANDA may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of LAZANDA and know how they will react to the medication.
-
ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials of a drug product cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of LAZANDA has been evaluated in a total of 523 opioid-tolerant patients with breakthrough cancer pain. The average duration of therapy in patients in the long-term study was 73 days, with 153 patients being treated for over 3 months. Patients continuing into the open-label extension period of the safety study have been treated for up to 26 months.
The clinical trials of LAZANDA were designed to evaluate safety and efficacy in treating breakthrough cancer pain; all patients were also taking concomitant opioids, such as sustained-release morphine, sustained-release oxycodone, or transdermal fentanyl, for their persistent cancer pain. The adverse reaction data presented in Table 1 reflect the actual percentage of patients experiencing each adverse effect among patients who received LAZANDA for breakthrough cancer pain along with a concomitant opioid for persistent cancer pain. There has been no attempt to correct for concomitant use of other opioids, duration of LAZANDA therapy, or cancer-related symptoms. Adverse events are included regardless of causality or severity. Table 1 lists adverse reactions with an overall frequency of 5% or greater within the total population that occurred during titration by maximum dose received. The ability to assign LAZANDA a dose-response relationship to these adverse events is limited by the titration schemes used in these studies.
Table 1: Adverse Reactions That Occurred During Titration at a Frequency of >5%
System Organ Class MedDRA preferred term, n (%)
100 mcg
(N=483)
200 mcg
(N=380)
400 mcg
(N=301)
800 mcg
(N=161)
Total
(N=516)
Gastrointestinal disorders
Nausea
19 (4)
6 (2)
6 (2)
5 (3)
35 (7)
Vomiting
14 (3)
10 (3)
9 (3)
1 (1)
33 (6)
Nervous system disorders
Dizziness
14 (3)
11 (3)
6 (2)
4 (2)
31 (6)
Table 2 lists, by dose, adverse reactions with an overall frequency of ≥5% within the total population that occurred after a final titrated dose had been determined.
Table 2: Adverse Reactions That Occurred During Maintenance Treatment at a Frequency of ≥5%
System Organ Class MedDRA preferred term, n (%)
100 mcg
(N=61)
200 mcg
(N=68)
400 mcg
(N=109)
800 mcg
(N=108)
Total
(N=346)
Gastrointestinal disorders
Vomiting
8 (13)
5 (7)
9 (8)
12 (11)
34 (10)
Nausea
4 (7)
6 (9)
4 (4)
9 (8)
23 (7)
Constipation
6 (10)
1 (1)
8 (7)
5 (5)
20 (6)
General disorders and administration site conditions
Pyrexia
3 (5)
5 (7)
8 (7)
6 (6)
22 (6)
The adverse reactions listed below represent those that occurred in ≥1% of patients from clinical trials while receiving LAZANDA. Events are classified by system organ class.
Eye disorders: dry eye, swelling, ptosis, strabismus
Blood and Lymphatic System Disorders: anemia, neutropenia
Cardiac Disorders: cardiorespiratory arrest
Gastrointestinal Disorders: vomiting, nausea, constipation, diarrhea, abdominal pain, gastritis, ascites, dry mouth, dyspepsia, mouth ulcer, proctalgia
General Disorders and Administration Site Conditions: pyrexia, fatigue, edema peripheral, asthenia, edema
Hepatobiliary Disorders: jaundice
Immune System Disorders: hypersensitivity
Infections and Infestations: urinary tract infection, pneumonia, nasopharyngitis, infection, rhinitis, upper respiratory tract infection, bronchitis
Injury, Poisoning and Procedural Complications: fall
Investigations: weight decreased, blood alkaline phosphatase increased
Metabolism and Nutrition Disorders: dehydration, decreased appetite, hyperglycemia, anorexia
Musculoskeletal and Connective Tissue Disorders: back pain, pain in extremity, arthralgia
Nervous System Disorders: dizziness, somnolence, headache, dysgeusia
Psychiatric Disorders: anxiety, insomnia, depression, confusional state, disorientation, agitation
Respiratory, Thoracic and Mediastinal Disorders: dyspnea, epistaxis, cough, pharyngolaryngeal pain, nasal discomfort, rhinorrhea, nasal congestion, postnasal drip, pulmonary embolism
Skin and Subcutaneous Tissue Disorders: pruritus, hyperhidrosis, decubitus ulcer, mouth ulceration
Vascular Disorders: hypertension, deep vein thrombosis
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use fentanyl. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in LAZANDA.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology (12.2)].
-
DRUG INTERACTIONS
Table 3 includes clinically significant drug interactions with LAZANDA.
Table 3: Clinically Significant Drug Interactions with LAZANDA
Inhibitors of CYP3A4
Clinical Impact:
The concomitant use of LAZANDA and CYP3A4 inhibitors can increase the plasma concentration of fentanyl, resulting in increased or prolonged opioid effects, particularly when an inhibitor is added after a stable dose of LAZANDA is achieved [see Warnings and Precautions ( 5.4)].
After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the fentanyl plasma concentration will decrease [see Clinical Pharmacology ( 12.3)], resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to fentanyl.
Intervention:
If concomitant use is necessary, consider dosage reduction of LAZANDA until stable drug effects are achieved. Monitor patients for respiratory depression and sedation at frequent intervals.
If a CYP3A4 inhibitor is discontinued, consider increasing the LAZANDA dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal.
Examples
Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g. ketoconazole), protease inhibitors (e.g., ritonavir), grapefruit juice
CYP3A4 Inducers
Clinical Impact:
The concomitant use of LAZANDA and CYP3A4 inducers can decrease the plasma concentration of fentanyl [see Clinical Pharmacology (12.3)], resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence to fentanyl [see Warnings and Precautions (5.18)].
After stopping a CYP3A4 inducer, as the effects of the inducer decline, the fentanyl plasma concentration will increase [see Clinical Pharmacology (12.3)], which could increase or prolong both the therapeutic effects and adverse reactions, and may cause serious respiratory depression.
Intervention:
If concomitant use is necessary, consider increasing the LAZANDA dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider LAZANDA dosage reduction and monitor for signs of respiratory depression.
Examples
Rifampin, carbamazepine, phenytoin
Benzodiazepines and Other Central Nervous System (CNS) Depressants
Clinical Impact:
Due to additive pharmacologic effect, the concomitant use of benzodiazepines and other CNS depressants, including alcohol, can increase the risk of hypotension, respiratory depression, profound sedation, coma, and death.
Intervention:
Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation [see Warnings and Precautions (5.4)].
If concomitant use is warranted, consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration (2.2), Warnings and Precautions (5.1, 5.4, 5.6)].
Examples:
Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol.
Serotonergic Drugs
Clinical Impact:
The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome [see Warnings and Precautions 5.10].
Intervention:
If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue LAZANDA if serotonin syndrome is suspected.
Examples:
Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that effect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Monoamine Oxidase Inhibitors (MAOIs)
Clinical Impact:
MAOI interactions with opioids may manifest as serotonin syndrome [see Warnings and Precautions (5.10)] or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.1)].
Intervention:
The use of LAZANDA is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
Examples:
phenelzine, tranylcypromine, linezolid
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics
Clinical Impact:
May reduce the analgesic effect of LAZANDA and/or precipitate withdrawal symptoms.
Intervention:
Avoid concomitant use.
Examples:
butorphanol, nalbuphine, pentazocine, buprenorphine,
Muscle Relaxants
Clinical Impact:
fentanyl may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
Intervention:
Monitor patients for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of LAZANDA and/or the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration ( 2.2), Warnings and Precautions ( 5.1, 5.4)]
Examples: cyclobenzaprine, metaxalone Diuretics
Clinical Impact:
Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone.
Intervention:
Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed.
Anticholinergic Drugs
Clinical Impact:
The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
Intervention:
Monitor patients for signs of urinary retention or reduced gastric motility when LAZANDA is used concomitantly with anticholinergic drugs.
Agents used to treat Allergic Rhinitis
Clinical Impact:
The presence of allergic rhinitis is not expected to affect LAZANDA absorption. However, co-administration of a vasoconstrictive nasal decongestant such as oxymetazoline to treat allergic rhinitis leads to lower peak plasma concentrations and a delayed T max of fentanyl that may cause LAZANDA to be less effective in patients with allergic rhinitis who use such decongestants, thus potentially impairing pain management. Additionally, in view of the possibility that the titration of a patient while they are experiencing an acute episode of rhinitis could lead to incorrect dose identification (particularly if they are using a vasoconstrictive decongestant), titration under these circumstances must be avoided [see Clinical Pharmacology (12.3)].
Intervention:
Avoid using LAZANDA in patients with allergic rhinitis and consider other products with a different route of administration.
-
USE IN SPECIFIC POPULATIONS
2
8.1 Pregnancy
Risk Summary
Prolonged use of opioid analgesics during pregnancy may cause neonatal opioid withdrawal syndrome. Available data with LAZANDA in pregnant women are insufficient to inform a drug-associated risk for major birth defects and miscarriage.
In animal reproduction studies fentanyl administration to pregnant rats during organogenesis was embryocidal at doses within the range of the human recommended dosing for LAZANDA. No evidence of malformations were noted in animal studies completed to date [ see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Prolonged use of opioid analgesics during pregnancy for medical or nonmedical purposes can result in physical dependence in the neonate and neonatal opioid withdrawal syndrome shortly after birth.
Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn. Observe newborns for symptoms of neonatal opioid withdrawal syndrome and manage accordingly [see Warnings and Precautions (5.3)].
Labor or Delivery
Opioids cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. An opioid antagonist, such as naloxone, must be available for reversal of opioid-induced respiratory depression in the neonate. LAZANDA is not recommended for use in pregnant women during or immediately prior to labor, when other analgesic techniques are more appropriate. Opioid analgesics, including LAZANDA, can prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilation, which tends to shorten labor. Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Data
Human Data
In women treated acutely with intravenous or epidural fentanyl during labor, symptoms of neonatal respiratory or neurological depression were no more frequent than would be expected in infants of untreated mothers.
Transient neonatal muscular rigidity has been observed in infants whose mothers were treated with intravenous fentanyl.
Animal Data
Fentanyl has been shown to embryocidal in pregnant rats at doses of 30 mcg/kg intravenously (0.4 times the 800 mcg dose of LAZANDA on a mg/m 2 basis) and 160 mcg/kg subcutaneously (2 times the 800 mcg dose of LAZANDA based on a mg/m 2 basis). There was no evidence of teratogenicity reported.
No evidence of malformations or adverse effects on the fetus was reported in a published study in which pregnant rats were administered fentanyl continuously via subcutaneously implanted osmotic minipumps at doses of 10, 100, or 500 mcg/kg/day starting 2-weeks prior to breeding and throughout pregnancy. The high dose was approximately 6 times the human dose of 800 mcg LAZANDA per pain episode on a mg/m 2 basis and produced mean steady-state plasma levels that are 3 times higher than the mean C max observed following administration of 800 mcg dose of LAZANDA in humans.
8.2 Lactation
Risk Summary
Fentanyl is present in breast milk. One published lactation study reports a relative infant dose of fentanyl of 0.024%. However, there is insufficient information to determine the effects of fentanyl on the breastfed infant and the effects of fentanyl on milk production.
Because of the potential for serious adverse reactions, including excess sedation and respiratory depression in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with LAZANDA.
Clinical Considerations
Monitor infants exposed to LAZANDA through breast milk for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
8.3 Females and Males of Reproductive Potential
Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Adverse Reactions (6.2), Clinical Pharmacology (12.2), Nonclinical Toxicology(13.1)].
8.4 Pediatric Use
The safety and efficacy of LAZANDA have not been established in patients below the age of 18 years.
8.5 Geriatric Use
Of the 523 opioid tolerant cancer patients with breakthrough cancer pain in clinical studies of LAZANDA, 148 (28%) were aged 60 years and over. No clinically meaningful difference was noted in the safety profile of the group aged over 60 years versus that of younger patients in LAZANDA clinical trials.
Elderly patients have been shown to be more sensitive to the effects of fentanyl when administered intravenously compared with the younger population. Therefore, exercise caution when individually titrating LAZANDA in elderly patients to provide adequate efficacy while minimizing risk.
Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of LAZANDA slowly in geriatric patients and monitor closely for signs of central nervous system and respiratory depression [see Warnings and Precautions ( 5.1)].
Fentanyl is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Patients with Renal or Hepatic Impairment
Insufficient information exists to make recommendations regarding the use of LAZANDA in patients with impaired renal or hepatic function. Fentanyl is metabolized primarily via the human CYP3A4 isoenzyme system and the inactive metabolite is mostly eliminated in urine. If the drug is used in these patients, it is to be used with caution because of the hepatic metabolism and renal excretion of fentanyl.
It is recommended that LAZANDA be titrated to clinical effect for all patients with special care taken in patients with severe renal or hepatic disease.
8.7 Sex
Both male and female opioid-tolerant cancer patients were studied for the treatment of breakthrough cancer pain. No clinically relevant sex differences were observed in adverse events.
8.8 Patients with Allergic (Seasonal) Rhinitis
The pharmacokinetic and safety profiles of LAZANDA in individuals with known allergic (seasonal) rhinitis showed no clinically meaningful differences in rate or extent of exposure to fentanyl, or in local tolerability of LAZANDA when compared to Asymptomatic (Unchallenged) state. However, when treated for their rhinitis with oxymetazoline, LAZANDA absorption was compromised [see Pharmacokinetics ( 12.3)].
-
DRUG ABUSE AND DEPENDENCE
9.2 Abuse
LAZANDA contains fentanyl, a substance with a high potential for abuse similar to other opioids including hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone, and tapentadol. LAZANDA can be abused and is subject to misuse, addiction, and criminal diversion [see Warnings and Precautions ( 5.6)].
All patients treated with opioids require careful monitoring for signs of abuse and addiction, since use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Prescription drug abuse is the intentional non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects.
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal.
“Drug-seeking” behavior is very common in persons with substance use disorders. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing, or referral, repeated “loss” of prescriptions, tampering with prescriptions, and reluctance to provide prior medical records or contact information for other treating health care provider(s). “Doctor shopping” (visiting multiple prescribers to obtain additional prescriptions) is common among drug abusers and people suffering from untreated addiction. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Health care providers should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction.
LAZANDA, like other opioids, can be diverted for non-medical use into illicit channels of distribution. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests, as required by state and federal law, is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Risks Specific to the Abuse of LAZANDA
LAZANDA is for intranasal transmucosal use only. Abuse of LAZANDA poses a risk of overdose and death. The risk is increased with concurrent abuse of LAZANDA with alcohol and other central nervous system depressants.
9.3 Dependence
Both tolerance and physical dependence can develop during chronic opioid therapy. Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical dependence results in withdrawal symptoms after abrupt discontinuation or a significant dosage reduction of a drug. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity (e.g., naloxone, nalmefene), mixed agonist/antagonist analgesics (e.g., pentazocine, butorphanol, nalbuphine), or partial agonists (e.g., buprenorphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued opioid usage.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal signs [see Use in Specific Populations ( 8.1)].
-
OVERDOSAGE
Clinical Presentation
Acute overdose with LAZANDA can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Clinical Pharmacology (12.2)].
Treatment of Overdose
In case of overdose, priorities are: the reestablishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support techniques.
Opioid antagonists, such as naloxone, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to fentanyl overdose, administer an opioid antagonist.
Because the duration of opioid reversal is expected to be less than the duration of action of fentanyl in LAZANDA, carefully monitor the patient until spontaneous respiration is reliably re-established. If the response to an opioid antagonist is suboptimal or only brief in nature, administer additional antagonist as directed by the product’s prescribing information.
In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.
-
DESCRIPTION
LAZANDA (fentanyl) nasal spray is a liquid formulation of fentanyl citrate, an opioid agonist, intended for intranasal transmucosal administration. The product consists of a practically clear to clear, colorless, aqueous solution of fentanyl citrate in a glass multidose container to which is attached a metered-dose nasal spray pump with a visual and audible spray counter. Each actuation is designed to deliver a spray of 100 mcL of solution containing 100 mcg, 300 mcg or 400 mcg fentanyl base, respectively. This enables doses of 100 mcg , 300 mcg or 400 mcg to be administered using a single spray into one nostril (1 spray) and 200 mcg, 600 mcg or 800 mcg to be administered using a single spray into both nostrils (2 sprays).
Active ingredient: Fentanyl citrate, USP is N-(l-phenethyl-4-piperidyl) propionanilide citrate (1:1). Fentanyl is a highly lipophilic compound (octanol-water partition coefficient at pH 7.4 is 816:1). Fentanyl citrate is sparingly soluble in water (1:40). The molecular weight of the free base and citrate salt are 336.5 and 528.6, respectively. The pKa is 8.4. The compound has the following structural formula:
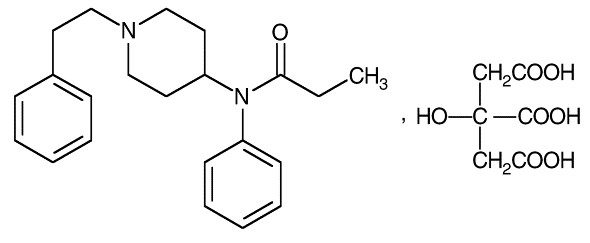
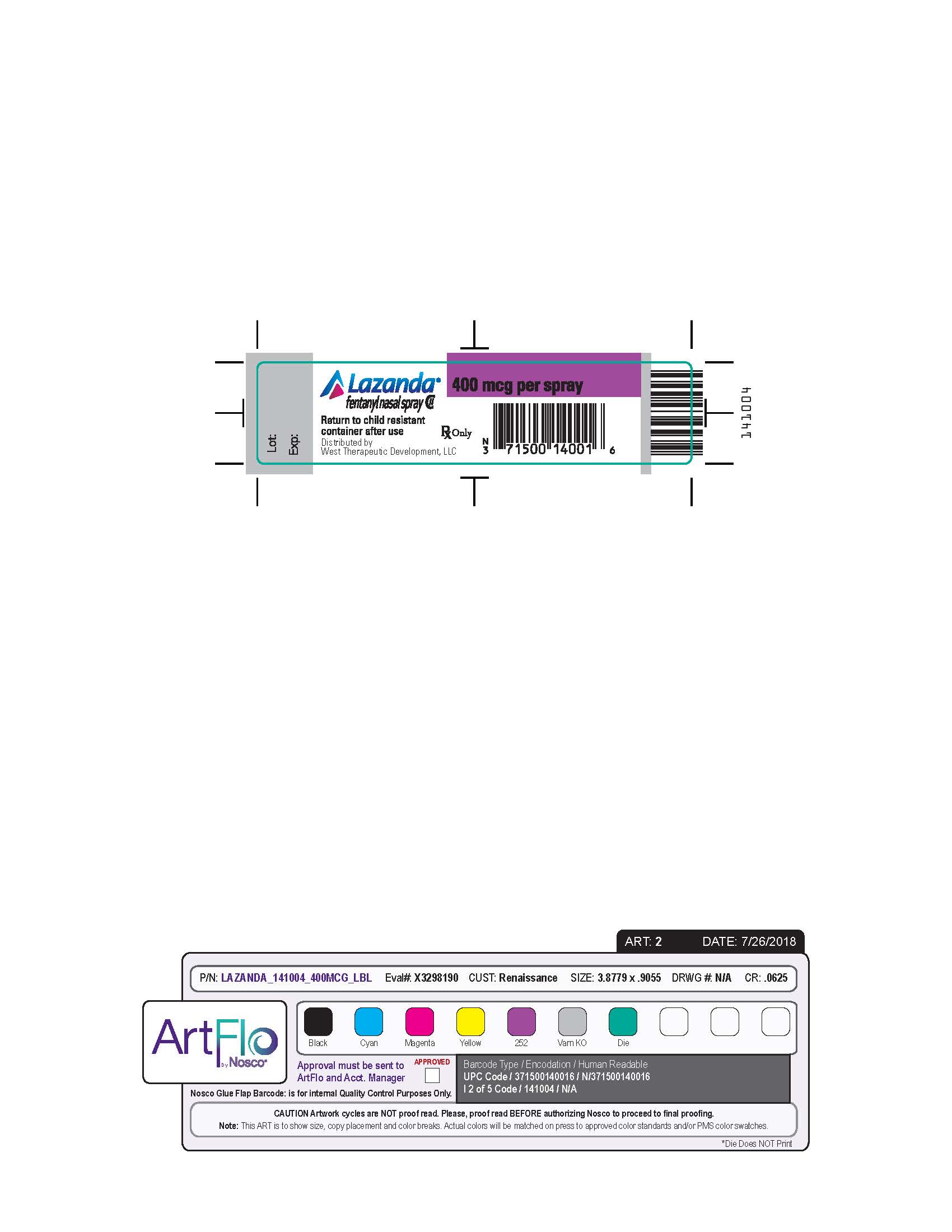
LAZANDA is available in 3 strengths of nasal spray: 100 mcg fentanyl (yellow label), 300 mcg fentanyl (blue label) and 400 mcg fentanyl (violet label). The strength is expressed as the amount of fentanyl free base per spray, e.g., the 100 mcg strength provides 100 mcg of fentanyl free base per 100 mcL spray.
Inactive ingredients: mannitol, pectin, phenylethyl alcohol, propylparaben, sucrose, water. Sodium hydroxide and/or hydrochloric acid are added if required for pH adjustment.
-
CLINICAL PHARMACOLOGY SECTION
12.1 Mechanism of Action
Fentanyl is an opioid agonist whose principal therapeutic action is analgesia.
12.2 Pharmacodynamics
Effects on the Central Nervous System
Fentanyl produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increase in carbon dioxide tension and electrical stimulation.
Fentanyl causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Fentanyl causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Fentanyl produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating and/or orthostatic hypotension.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans [see Adverse Reactions ( 6.2)]. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see Adverse Reactions ( 6.2)].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The analgesic effects of fentanyl are related to the blood level of the drug, if proper allowance is made for the delay into and out of the CNS (a process with a 3- to 5-minute half-life).
In general, the effective concentration and the concentration at which toxicity occurs increase with increasing tolerance with any and all opioids. The rate of development of tolerance varies widely among individuals.
The minimum effective analgesic concentration of fentanyl for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome and/or the development of analgesic tolerance [see Dosage and Administration ( 2.1, 2.5)].
Concentration–Adverse Reaction Relationships
There is a relationship between increasing fentanyl plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration ( 2.1, 2.2, 2.3)].
Respiratory System
All opioid mu-receptor agonists, including fentanyl, produce dose-dependent respiratory depression. The risk of respiratory depression is less in patients receiving chronic opioid therapy who develop tolerance to respiratory depression and other opioid effects. Peak respiratory depressive effects may be seen as early as 15 to 30 minutes from the start of transmucosal fentanyl citrate product administration and may persist for several hours.
Serious or fatal respiratory depression can occur even at recommended doses. Although not observed with oral or nasal transmucosal fentanyl products in clinical trials, fentanyl given rapidly by intravenous injection in large doses may interfere with respiration by causing rigidity in the muscles of respiration. [see Warnings and Precautions ( 5.1), Adverse Reactions ( 6), Overdosage ( 10)].
12.3 Pharmacokinetics
Absorption
In a study that compared the relative bioavailability of LAZANDA and an oral transmucosal fentanyl citrate product, the bioavailability of fentanyl from LAZANDA was approximately 20% higher. Fentanyl is absorbed from the nasal mucosa following intranasal administration of LAZANDA, with median T max values ranging from 15-21 min after administration of a single dose. C max and AUC values for fentanyl following administration of LAZANDA increase linearly over the 100- to 800-mcg dose range.
Mean plasma concentration versus time profiles are presented in Figure 1. Mean pharmacokinetic parameters are presented in Table 4.
Figure 1.
Mean Plasma Fentanyl Concentration (pg/mL) in Normal Subjects Receiving 100, 200, 400 and 800 mcg Lazanda or 200 mcg OTFC
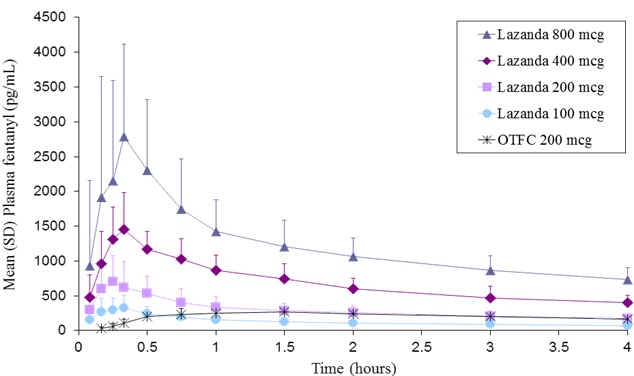
Table 4. Pharmacokinetic Parameters in Normal Subjects Receiving 100, 200, 400, and 800 mcg of LAZANDA or 200 mcg OTFC
LAZANDA
OTFC
PHARMACOKINETIC PARAMETERS
100 mcg
200 mcg
400 mcg
800 mcg
200 mcg
T max, hours
median (range)
0.33
(0.08–1.50)
0.25
(0.17–1.60)
0.35
(0.25–0.75)
0.34
(0.17–3.00)
1.50
(0.50–8.00)
C max, pg/mL
Mean (%CV)
351.5
(51.3)
780.8
(48.4)
1552.1
(26.2)
2844.0
(56.0)
317.4
(29.9)
AUC inf, pg.hour/mL
Mean (%CV)
2460.5
(17.9)
4359.9
(29.8)
7513.4
(26.7)
17272
(48.9)
3735.0
(32.8)
t 1/2, hour
Mean (%CV)
21.9
(13.6)
24.9
(51.3)
15.0
(24.7)
24.9
(92.5)
18.6
(31.4)
A pharmacokinetic study evaluated the cerebrospinal fluid (CSF) concentrations of fentanyl administered via the intranasal (LAZANDA) route. As presented in Table 5, the maximum concentration of fentanyl in the CSF as delivered by LAZANDA was reached at 1.0 hour. Values for the C max and AUC 0-6h of fentanyl are also presented in Table 5.
Table 5 Cerebrospinal Pharmacokinetic Parameters in Normal Subjects Receiving 200 mcg of LAZANDA
PHARMACOKINETICS PARAMETERS
LAZANDA
(fentanyl nasal spray)
200 mcgT max, hours
Median (range)
1.01
(0.75–3.00)C max, pg /mL
Mean (%CV)
84.54
(55.7)
AUC 0-6h, pg.hour/mL
Mean (%CV)
300.25
(40.6)
In a pharmacokinetic study that evaluated multiple-dose pharmacokinetics of LAZANDA when two doses of LAZANDA are administered in the same nostril and are separated by a 1, 2 or 4 h time lapse, C max2 (C max after second administration) was greater than C max1 (C max after first administration), by 30% when LAZANDA was administered 1 h apart, by 25% when LAZANDA was administered 2 h apart and by 10% when LAZANDA was administered 4 h apart. Based on these results and based on T max range of LAZANDA observed across pharmacokinetic studies, and frequency of breakthrough pain episodes in a cancer population, a waiting period of 2 h between two consecutive doses of LAZANDA is recommended [see Dosage and Administration (2.3, 2.4)].
In a pharmacokinetic study to evaluate differences in LAZANDA absorption in individuals with induced allergic (seasonal) rhinitis using Ragweed, no clinically meaningful differences were observed in rate or extent of exposure to fentanyl, when compared to the Asymptomatic (Unchallenged) state, indicating that presence of allergic rhinitis does not affect LAZANDA absorption. This study also assessed differences in Lazanda absorption, if any, when co-administered with oxymetazoline, a nasal decongestant in subjects undergoing treatment for seasonal allergic rhinitis. The mean C max and AUC t values for Treated arm (Rhinitis treated with oxymetazoline) were about 32% and 10% lower , respectively compared to the Asymptomatic arm. In addition, mean T max of LAZANDA in the Treated arm was 0.75 h (range 0.08-3 h) as compared to 0.25 h (0.17-1 h) for the Asymptomatic arm. These results indicate that co-administration with oxymetazoline in rhinitis leads to lower peak plasma concentrations and delayed T max of LAZANDA [see Drug Interactions (7)].
Distribution
Fentanyl is highly lipophilic. The plasma protein binding of fentanyl is 80% to 85%. The main binding protein is alpha-1-acid glycoprotein, but both albumin and lipoproteins contribute to some extent. The mean volume of distribution at steady state (Vss) was 4 L/kg.
Elimination
The total plasma clearance of fentanyl following intravenous administration is approximately 42 L/h.
Metabolism
The metabolic pathways following intranasal administration of LAZANDA have not been characterized in clinical studies. The progressive decline of fentanyl plasma concentrations results from the uptake of fentanyl in the tissues and biotransformation in the liver. Fentanyl is metabolized in the liver and in the intestinal mucosa to norfentanyl by cytochrome P450 3A4 isoform. In animal studies, norfentanyl was not found to be pharmacologically active.
Excretion
The disposition of fentanyl following intranasal administration of LAZANDA has not been characterized in a mass balance study. Fentanyl is primarily (more than 90%) eliminated by biotransformation to N-dealkylated and hydroxylated inactive metabolites. Less than 7% of the administered dose is excreted unchanged in the urine, and only about 1% is excreted unchanged in the feces. The metabolites are mainly excreted in the urine, while fecal excretion is less important.
-
NONCLINICAL TOXICOLOY
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of fentanyl have not been conducted.
Mutagenesis
Fentanyl citrate was not mutagenic in the in vitro Ames reverse mutation assay in S. typhimurium or E. coli, or the mouse lymphoma mutagenesis assay, and was not clastogenic in the in vivo mouse micronucleus assay.
Impairment of Fertility
Fentanyl has been shown to impair fertility in rats at doses of 30 mcg/kg subcutaneously. Conversion to human equivalent doses indicates this is within the range of the human recommended dosing for LAZANDA.
-
CLINICAL STUDIES
The efficacy of LAZANDA was evaluated in one clinical trial in opioid tolerant adult patients experiencing breakthrough cancer pain. Breakthrough cancer pain was defined as a transient flare of moderate-to-severe pain occurring in patients experiencing persistent cancer pain otherwise controlled with maintenance doses of opioid medications including at least 60 mg of oral morphine/day or an equianalgesic dose of another opioid (which could be fentanyl) for a week or longer. All patients were on stable doses of either long-acting oral opioids or transdermal fentanyl for their persistent cancer pain.
The clinical trial included an open-label titration phase where a dose was identified that provided adequate analgesia with tolerable side effects, within the range of 100 to 800 mcg. In the double-blind, placebo-controlled portion of the study, patients who were titrated to an adequate dose were randomized to a blinded sequence of 10 treatments with 7 being the identified dose of LAZANDA and 3 being placebo.
Of the patients who enrolled in the study, 73% achieved an adequate dose during the titration phase, 6% withdrew for lack of effective pain relief, and 5% withdrew due to adverse events.
The distribution of final titrated doses is shown in Table 5. The final titrated dose of LAZANDA for breakthrough pain was not predicted from the daily maintenance dose of opioid used to manage the persistent cancer pain and, therefore, the dose was determined by titration starting at 100 mcg.
Table 6. Dose of LAZANDA Following Initial Titration (ITT population)
LAZANDA Dose
(N=83) n (%)
100 mcg
12 (14)
200 mcg
7 (8)
400 mcg
27 (33)
800 mcg
37 (45)
The primary outcome measure, the mean sum of the pain intensity difference at 30 minutes (SPID30), was statistically significantly higher for LAZANDA than for placebo (see Figure 2).
Figure 2.
Pain Intensity Differences (PID) following LAZANDA or Placebo in Adult Patients with Breakthrough Cancer Pain
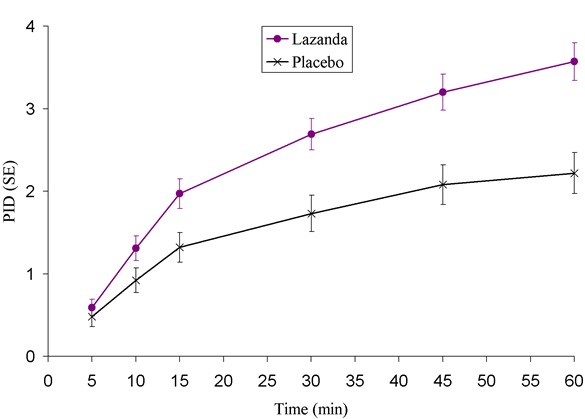
-
HOW SUPPLIED
LAZANDA is available as a 5.3 mL capacity clear glass bottle with an attached metered-dose nasal spray pump incorporating a visual and audible spray counter, and a protective dust cover. Each glass bottle, contains 8 sprays of 100 mcL available in three different concentrations: 100 mcg/100 mcL, 300 mcg/100 mcL or 400 mcg/100 mcL concentration solution. Each bottle contains a net fill weight of 1.57 grams and, after priming, delivers 8 sprays.
The pump will remain primed for up to 5 days after priming or use. If the product has not been used for 5 days, re-prime by spraying once. The nasal spray delivers 8 full sprays. There are 3 product strengths and each 100 mcL spray contains either 100 mcg, 300 mcg or 400 mcg of fentanyl. Each bottle is supplied in a child-resistant container.
Bottles in their child-resistant containers are supplied in cartons containing 1 bottle with instructions for use.
Each carton contains one carbon-lined pouch per bottle for disposal of priming sprays, unwanted doses and residual fentanyl solution.
Lazanda Dosage Strength
(fentanyl base)
Number of Bottles per Carton NDC Number 100 mcg 1 71500-110-01 300 mcg 1 71500-130-01 400 mcg 1 71500-140-01 Store at up to 25°C. Do not freeze.
Note: Carton and bottle label colors are a secondary aid in product identification. Confirm the printed dosage before dispensing.
Store LAZANDA securely and dispose of properly [see Patient Counseling Information (17)].
-
PATIENT COUNSELING
Storage and Disposal of Unused LAZANDA [see Instructions for Use]
- Because of the risks associated with accidental ingestion, misuse, and abuse, advise patients to store LAZANDA securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home [see Warnings and Precautions (5.2, 5.6), Drug Abuse and Dependence (9.2)]. Inform patients that leaving LAZANDA unsecured can pose a deadly risk to others in the home.
- Advise patients and caregivers to return the bottle to the child-resistant container after each use. Put the bottle in its child-resistant container and the pouch in the cardboard carton and store securely out of the reach of children and protect from light.
- Advise patients and caregivers that when medicines are no longer needed, they should be disposed of promptly. Inform patients that they can visit www.fda.gov/drugdisposal for a complete list of medicines recommended for disposal by flushing, as well as additional information on disposal of unused medicines.
- Instruct patients that, to dispose of LAZANDA properly, the remaining liquid in all bottles must be sprayed into the pouch provided in the pack for safe disposal as soon as possible. This includes any unwanted therapeutic sprays remaining in the bottle. After the counter has advanced to “8”, the patient should continue to push down on the finger grips a total of four times in order to expel any residual medicine from the bottle. After the 8 therapeutic sprays have been emitted, the patient will not hear a click and the counter will not advance beyond “8”; further sprays emitted will not be full sprays and should always be trapped in the pouch, not used therapeutically.
- Instruct the patient and caregiver to seal the pouch and to place both the empty bottle and the sealed pouch into the child-resistant storage container and discard in the trash. LAZANDA must be stored in the specially provided child-resistant container out of the reach of children until proper disposal is possible.
- Instruct the patient and caregiver to wash their hands with soap and water immediately after handling the pouch.
- If the pouch is lost, instruct the patient and caregiver to use a pouch from another LAZANDA pack to prime and dispose of unused medicine from the current bottle as well as from the next bottle. If they do not have an empty pouch available, the patient or caregiver can order one by calling 1-844-452-9263. They will receive the replacement pouch in the mail.
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Life-Threatening Respiratory Depression
Inform patients of the risk of life-threatening respiratory depression, including information that the risk is greatest when starting LAZANDA or when the dosage is increased, and that it can occur even at recommended dosages.
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Warnings and Precautions ( 5.1)].
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss with the patient and caregiver the availability of naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with LAZANDA. Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program) [see Dosage and Administration ( 2.2), Warnings and Precautions ( 5.1)].
Educate patients and caregivers on how to recognize the signs and symptoms of an overdose.
Explain to patients and caregivers that naloxone’s effects are temporary, and that they must call 911 or get emergency medical help right away in all cases of known or suspected opioid overdose, even if naloxone is administered [see Overdosage ( 10)].
If naloxone is prescribed, also advise patients and caregivers:
- How to treat with naloxone in the event of an opioid overdose
- To tell family and friends about their naloxone and to keep it in a place where family and friends can access it in an emergency
- To read the Patient Information (or other educational material) that will come with their naloxone. Emphasize the importance of doing this before an opioid emergency happens, so the patient and caregiver will know what to do.
Increased Risk of Overdose and Death in Children Due to Accidental Exposure
- Healthcare providers and dispensing pharmacists must specifically question patients or caregivers about the presence of children in the home (on a full time or visiting basis) and counsel them regarding the dangers to children from inadvertent exposure [see Warnings and Precautions (5.2)].
- Inform patients and their caregivers that accidental exposure, especially in children, may result in respiratory depression or death [see Warnings and Precautions (5.2)].
- Instruct patients and their caregivers that in the event that a LAZANDA unit is not completely consumed, it must be properly disposed as soon as possible [see Dosage and Administration (2.8), Patient Counseling Information; Disposal of Unused LAZANDA (17)].
- Instruct patients and caregivers to keep both used and unused LAZANDA out of the reach of children [see Warnings and Precautions (5.2)].
Interactions with Benzodiazepines and Other CNS Depressants
Inform patients that potentially fatal additive effects may occur if LAZANDA is used with benzodiazepines or other CNS depressants, including alcohol, and not to use these concomitantly unless supervised by a health care provider [see Warnings and Precautions (5.4), Drug Interactions (7)]
Addiction, Abuse, and Misuse
Inform patients that the use of LAZANDA, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose and death [see Warnings and Precautions (5.6)]. Instruct patients not to share LAZANDA with others and to take steps to protect LAZANDA from theft or misuse.
Transmucosal Immediate-Release Fentanyl (TIRF) REMS
LAZANDA is available only through a restricted program called the Transmucosal Immediate Release Fentanyl (TIRF) REMS [see Warnings and Precautions (5.7)]. Inform the patient of the following notable requirements:
- Outpatients must be enrolled in the REMS program
- Patients must be opioid-tolerant to receive LAZANDA
LAZANDA is available only from certified pharmacies participating in this program. Therefore, provide patients with the telephone number and website for information on how to obtain the product.
Pharmacies, outpatients, and healthcare professionals who prescribe to outpatients are required to enroll in the program. Inpatient pharmacies must develop policies and procedures to verify opioid tolerance in inpatients who require LAZANDA while hospitalized [see Warnings and Precautions (5.7)].
Serotonin Syndrome
Inform patients that opioids could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their physicians if they are taking, or plan to take serotonergic medications. [see Warnings and Precautions (5.10), Drug Interactions (7)].
MAOI Interaction
Inform patients to avoid taking LAZANDA while using any drugs that inhibit monoamine oxidase. Patients should not start MAOIs while taking LAZANDA [see Warnings and Precautions (5.10); Drug Interactions (7)].
Adrenal Insufficiency
Inform patients that opioids could cause adrenal insufficiency, a potentially life-threatening condition. Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Advise patients to seek medical attention if they experience a constellation of these symptoms [see Warnings and Precautions (5.11)].
Important Administration Instructions [see Dosage and Administration (2)]
- Instruct patients not to take LAZANDA for acute pain, postoperative pain, pain from injuries, headache, migraine, or any other short-term pain, even if they have taken other opioid analgesics for these conditions.
- Instruct patients on the meaning of opioid tolerance and LAZANDA is only to be used as a supplemental pain medication for patients with pain requiring regular opioids, who have developed tolerance to the opioid medication and who need additional opioid treatment of breakthrough pain episodes.
- Instruct patients that if they are not taking an opioid medication on a regular around-the-clock basis, they should not take LAZANDA.
- Advise patients that LAZANDA contains fentanyl, which is a pain medication similar to hydromorphone, methadone, morphine, oxycodone, oxymorphone, hydrocodone, and tapentadol.
- Instruct patients that they MUST wait at least 2 hours before treating another episode of breakthrough pain with LAZANDA.
- Instruct patients to talk to their doctor if breakthrough pain is not alleviated or worsens after taking LAZANDA.
- Instruct patients to use LAZANDA exactly as prescribed by their doctor and not to take LAZANDA more often than prescribed.
- Instruct patients NOT to share LAZANDA and that sharing LAZANDA with anyone else could result in the other individual’s death due to overdose.
- Instruct patients and their caregivers that the amount of fentanyl contained in a bottle can be fatal to a child. Patients and their caregivers must be instructed to keep LAZANDA in its child-resistant container at all times and to store it and the pouch securely and out of the reach of children.
Hypotension
Inform patients that LAZANDA may cause orthostatic hypotension and syncope. Instruct patients how to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position) [see Warnings and Precautions (5.12)].
Anaphylaxis
Inform patients that anaphylaxis have been reported with ingredients contained in LAZANDA. Advise patients how to recognize such a reaction and when to seek medical attention [see Contraindications (4), Adverse Reactions (6.2)].
Pregnancy
Neonatal Opioid Withdrawal Syndrome
Inform patients that prolonged use of LAZANDA during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated [see Warnings and Precautions (5.8), Use in Specific Populations (8.1)].
Embryo-Fetal Toxicity
Inform female patients of reproductive potential that LAZANDA can cause fetal harm and to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise nursing mothers to monitor infants for increased sleepiness (more than usual), breathing difficulties, or limpness. Instruct nursing mothers to seek immediate medical care if they notice these signs [see Use in Specific Populations (8.2)].
Infertility
Inform patients that chronic use of opioids may cause reduced fertility. It is not known whether these effects on fertility are reversible [see Use in Specific Populations (8.3)].
Driving or Operating Heavy Machinery
Inform patients that LAZANDA may impair the ability to perform potentially hazardous activities such as driving a car or operating heavy machinery. Advise patients not to perform such tasks until they know how they will react to the medication [see Warnings and Precautions (5.16)].
Constipation
Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention [see Adverse Reactions (6.1)].
Manufactured for: West Therapeutic Development, LLC
Northbrook, IL 60062
For information about this product call:
1-844-452-9263
141011
Lazanda is a registered trademark of West Therapeutic Development, LLC
© 2020 West Therapeutic Development, LLC
March 2021
LAZ-005-C.5
- Medication Guide
- PRINCIPAL DISPLAY PANEL - POUCH LABEL
- PRINCIPAL DISPLAY PANEL - 100 MCG LABEL
-
PRINCIPAL DISPLAY PANEL - 100 MCG 1-BOTTLE CARTON
NDC 71500-110-01
100 mcg per spray
Lazanda®
fentanyl nasal spray CIIFor nasal administration only
Dispense the enclosed
Medication Guide to each patient1 bottle, containing 8 sprays, and 1 disposal pouch
WARNING:
Keep out of the reach of childrenPATIENTS MUST BE TOLERANT TO
AROUND-THE-CLOCK OPIOID THERAPYDO NOT SUBSTITUTE LAZANDA
FOR OTHER FENTANYL PRODUCTSLazanda can be harmful or fatal if
given to someone for whom it was
not prescribedStore the bottle in the child resistant
container at all times, even when finished.
- PRINCIPAL DISPLAY PANEL - 300 MCG LABEL
-
PRINCIPAL DISPLAY PANEL - 300 MCG 1-BOTTLE CARTON
NDC 71500-130-01
300 mcg per spray
Lazanda®
fentanyl nasal spray CIIFor nasal administration only
Dispense the enclosed
Medication Guide to each patient1 bottle, containing 8 sprays, and 1 disposal pouch
WARNING:
Keep out of the reach of childrenPATIENTS MUST BE TOLERANT TO
AROUND-THE-CLOCK OPIOID THERAPYDO NOT SUBSTITUTE LAZANDA
FOR OTHER FENTANYL PRODUCTSLazanda can be harmful or fatal if
given to someone for whom it was
not prescribedStore the bottle in the child resistant
container at all times, even when finished.
- PRINCIPAL DISPLAY PANEL - 400 MCG LABEL
-
PRINCIPAL DISPLAY PANEL - 400 MCG 1-BOTTLE CARTON
NDC 71500-140-01
400 mcg per spray
Lazanda®
fentanyl nasal spray CIIFor nasal administration only
Dispense the enclosed
Medication Guide to each patient1 bottle, containing 8 sprays, and 1 disposal pouch
WARNING:
Keep out of the reach of childrenPATIENTS MUST BE TOLERANT TO
AROUND-THE-CLOCK OPIOID THERAPYDO NOT SUBSTITUTE LAZANDA
FOR OTHER FENTANYL PRODUCTSLazanda can be harmful or fatal if
given to someone for whom it was
not prescribedStore the bottle in the child resistant
container at all times, even when finished.
-
INGREDIENTS AND APPEARANCE
LAZANDA
fentanyl sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71500-140 Route of Administration NASAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL CITRATE (UNII: MUN5LYG46H) (FENTANYL - UNII:UF599785JZ) FENTANYL 400 ug Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) PECTIN (UNII: 89NA02M4RX) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71500-140-01 1 in 1 CARTON 08/25/2018 1 8 in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022569 08/25/2018 LAZANDA
fentanyl sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71500-130 Route of Administration NASAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL CITRATE (UNII: MUN5LYG46H) (FENTANYL - UNII:UF599785JZ) FENTANYL 300 ug Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) PECTIN (UNII: 89NA02M4RX) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71500-130-01 1 in 1 CARTON 08/25/2018 1 8 in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022569 08/25/2018 LAZANDA
fentanyl sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71500-110 Route of Administration NASAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENTANYL CITRATE (UNII: MUN5LYG46H) (FENTANYL - UNII:UF599785JZ) FENTANYL 100 ug Inactive Ingredients Ingredient Name Strength PECTIN (UNII: 89NA02M4RX) HYPROMELLOSES (UNII: 3NXW29V3WO) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) PROPYLPARABEN (UNII: Z8IX2SC1OH) COPOVIDONE K25-31 (UNII: D9C330MD8B) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71500-110-01 1 in 1 CARTON 08/25/2018 1 8 in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022569 08/25/2018 Labeler - West Therapeutic Development LLC (080707245) Registrant - Renaissance Lakewood LLC (077744035) Establishment Name Address ID/FEI Business Operations Renaissance Lakewood LLC 077744035 label(71500-110, 71500-130, 71500-140) , pack(71500-110, 71500-130, 71500-140) , manufacture(71500-110, 71500-130, 71500-140)