Label: MOMETASONE FUROATE solution
- NDC Code(s): 45802-118-46, 45802-118-59
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MOMETASONE FUROATE TOPICAL SOLUTION safely and effectively. See full prescribing information for MOMETASONE FUROATE TOPICAL SOLUTION.
MOMETASONE FUROATE topical solution, for topical use
Initial U.S. Approval: 1987INDICATIONS AND USAGE
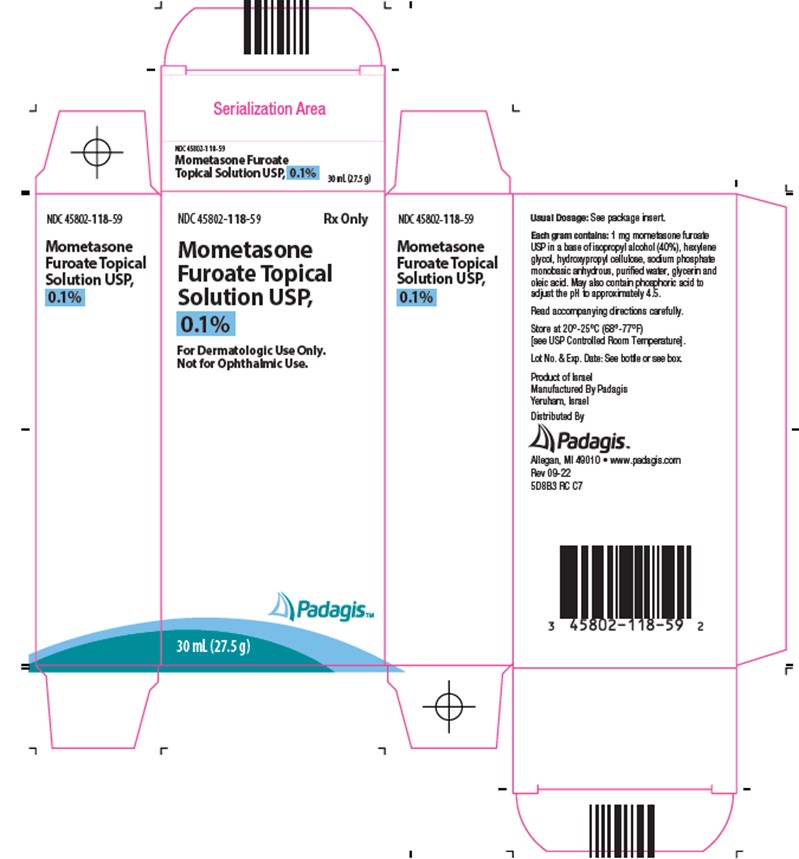
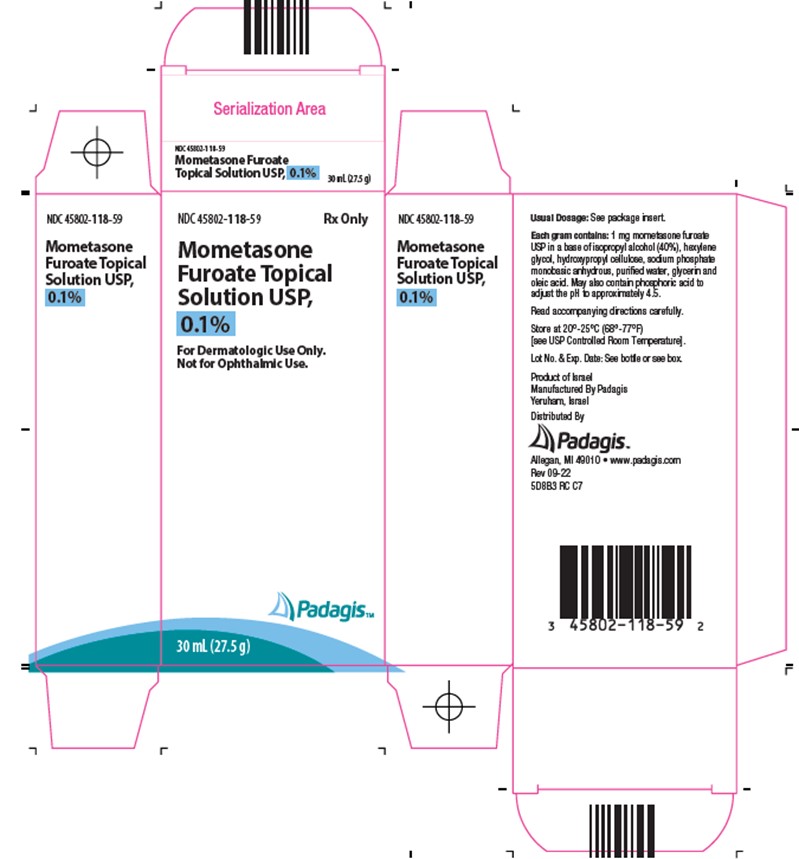
Mometasone Furoate Topical Solution 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients ≥ 12 years of age. (1)
DOSAGE AND ADMINISTRATION
- •
- Apply a few drops to the affected skin areas once daily and massage lightly until it disappears. (2)
- •
- Discontinue therapy when control is achieved. (2)
- •
- If no improvement is seen within 2 weeks, reassess diagnosis. (2)
- •
- Do not use with occlusive dressings unless directed by a physician. (2)
DOSAGE FORMS AND STRENGTHS
- •
- Solution, 0.1%. (3)
CONTRAINDICATIONS
- •
- Mometasone Furoate Topical Solution, 0.1% is contraindicated in those patients with a history of hypersensitivity to any of the components in the preparation. (4)
WARNINGS AND PRECAUTIONS
- •
- Reversible HPA axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment, Cushing’s syndrome, and hyperglycemia may occur due to systemic absorption. Patients applying a topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of HPA axis suppression. Modify use should HPA axis suppression develop. (5.1, 8.4)
- •
- Pediatric patients may be more susceptible to systemic toxicity. (5.1, 8.4)
- •
- May increase the risk of cataracts and glaucoma. If visual symptoms occur, consider referral to an ophthalmologist. (5.2)
ADVERSE REACTIONS
Most common adverse reactions included are acneiform reaction, burning, itching and folliculitis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Endocrine System
5.2 Ophthalmic Adverse Reactions
5.3 Allergic Contact Dermatitis
5.4 Concomitant Skin Infections
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply a few drops of Mometasone Furoate Topical Solution 0.1% to the affected skin areas once daily and massage lightly until it disappears.
Therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary [seeWarnings and Precautions (5.1) and Use in Specific Populations (8.4)].
Do not use Mometasone Furoate Topical Solution, 0.1% with occlusive dressings unless directed by a physician. Do not apply Mometasone Furoate Topical Solution, 0.1% in the diaper area if the patient requires diapers or plastic pants, as these garments constitute occlusive dressing.
Mometasone Furoate Topical Solution 0.1% is for topical use only. It is not for oral, ophthalmic, or intravaginal use.
Avoid use on the face, groin, or axillae.
Avoid contact with eyes. Wash hands after each application.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Effects on Endocrine System
Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency. This may occur during treatment or after withdrawal of treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of high potency steroids, large treatment surface areas, prolonged use, use of occlusive dressing, altered skin barrier, liver failure and young age.
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. This may be done by using the adrenocorticotropic hormone (ACTH) stimulation test.
In a study evaluating the effects of mometasone furoate lotion on the HPA axis, 15 mL were applied without occlusion twice daily (30 mL per day) for 7 days to 4 adult subjects with scalp and body psoriasis. At the end of treatment, the plasma cortisol levels for each of the 4 subjects remained within the normal range and changed little from baseline.
If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or to substitute a less potent corticosteroid. Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids.
Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios [seeUse in Specific Populations (8.4)].
5.2 Ophthalmic Adverse Reactions
Use of topical corticosteroids may increase the risk of posterior subcapsular cataracts and glaucoma. Cataracts and glaucoma have been reported in postmarketing experience with the use of topical corticosteroid products, including the topical mometasone products [see Adverse Reactions (6.2)].
Avoid contact of Mometasone Furoate Topical Solution, 0.1% with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
5.3 Allergic Contact Dermatitis
If irritation develops, Mometasone Furoate Topical Solution, 0.1% should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation. Such an observation should be corroborated with appropriate diagnostic patch testing.
5.4 Concomitant Skin Infections
If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of Mometasone Furoate Topical Solution 0.1% should be discontinued until the infection has been adequately controlled.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In clinical trials involving 209 subjects, the incidence of adverse reactions associated with the use of mometasone furoate lotion was 3%. Reported reactions included acneiform reaction, 2; burning, 4; and itching, 1. In an irritation/sensitization study involving 156 normal subjects, the incidence of folliculitis was 3% (4 subjects).
The following adverse reactions were reported to be possibly or probably related to treatment with mometasone furoate lotion during a clinical trial in 14% of 65 pediatric subjects 6 months to 2 years of age: decreased glucocorticoid levels, 4; paresthesia, 2; dry mouth,1; an unspecified endocrine disorder, 1; pruritus, 1; and an unspecified skin disorder, 1. The following signs of skin atrophy were also observed among 65 subjects treated with mometasone furoate lotion in a clinical trial: shininess, 4; telangiectasia, 2; loss of elasticity, 2; and loss of normal skin markings, 3.
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing reports for local adverse reactions to topical corticosteroids include irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, and miliaria. These adverse reactions may occur more frequently with the use of occlusive dressings.
Postmarketing reports for ophthalmic adverse reactions to topical corticosteroids include blurred vision, cataracts, glaucoma, increased intraocular pressure, and central serous chorioretinopathy.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects Pregnancy Category C:
There are no adequate and well-controlled studies in pregnant women. Therefore, Mometasone Furoate Topical Solution 0.1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
When administered to pregnant rats, rabbits, and mice, mometasone furoate increased fetal malformations. The doses that produced malformations also decreased fetal growth, as measured by lower fetal weights and/or delayed ossification. Mometasone furoate also caused dystocia and related complications when administered to rats during the end of pregnancy.
In mice, mometasone furoate caused cleft palate at subcutaneous doses of 60 mcg/kg and above. Fetal survival was reduced at 180 mcg/kg. No toxicity was observed at 20 mcg/kg. (Doses of 20, 60, and 180 mcg/kg in the mouse are approximately 0.01, 0.02, and 0.05 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis.)
In rats, mometasone furoate produced umbilical hernias at topical doses of 600 mcg/kg and above. A dose of 300 mcg/kg produced delays in ossification, but no malformations. (Doses of 300 and 600 mcg/kg in the rat are approximately 0.2 and 0.4 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis.)
In rabbits, mometasone furoate caused multiple malformations (e.g., flexed front paws, gallbladder agenesis, umbilical hernia, hydrocephaly) at topical doses of 150 mcg/kg and above (approximately 0.2 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis). In an oral study, mometasone furoate increased resorptions and caused cleft palate and/or head malformations (hydrocephaly and domed head) at 700 mcg/kg. At 2800 mcg/kg most litters were aborted or resorbed. No toxicity was observed at 140 mcg/kg. (Doses at 140, 700, and 2800 mcg/kg in the rabbit are approximately 0.2, 0.9, and 3.6 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis.)
When rats received subcutaneous doses of mometasone furoate throughout pregnancy or during the later stages of pregnancy, 15 mcg/kg caused prolonged and difficult labor and reduced the number of live births, birth weight, and early pup survival. Similar effects were not observed at 7.5 mcg/kg. (Doses of 7.5 and 15 mcg/kg in the rat are approximately 0.005 and 0.01 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis.)
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Mometasone Furoate Topical Solution 0.1% is administered to a nursing woman.
8.4 Pediatric Use
Since safety and efficacy of Mometasone Furoate Topical Solution 0.1% have not been established in pediatric patients below 12 years of age, its use in this age group is not recommended.
Mometasone furoate lotion caused HPA axis suppression in approximately 29% of pediatric subjects ages 6 to 23 months, who showed normal adrenal function by Cortrosyn test before starting treatment, and were treated for approximately 3 weeks over a mean body surface area of 40% (range 16%-90%). The criteria for suppression were: basal cortisol level of ≤5 mcg/dL, 30-minute post-stimulation level of ≤18 mcg/dL, or an increase of <7 mcg/dL. Follow-up testing 2 to 4 weeks after stopping treatment, available for 8 of the subjects, demonstrated suppressed HPA axis function in 1 subject, using these same criteria. Long-term use of topical corticosteroids has not been studied in this population [seeClinical Pharmacology (12.2)].
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are, therefore, also at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. Pediatric patients may be more susceptible than adults to skin atrophy, including striae, when they are treated with topical corticosteroids. Pediatric patients applying topical corticosteroids to greater than 20% of body surface are at higher risk of HPA axis suppression.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Mometasone Furoate Topical Solution 0.1% should not be used in the treatment of diaper dermatitis.
8.5 Geriatric Use
Clinical trials of mometasone furoate lotion did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious usually starting at the low end of the dosing range.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Mometasone Furoate Topical Solution USP, 0.1% contains mometasone furoate for topical use. Mometasone furoate is a synthetic corticosteroid with anti-inflammatory activity.
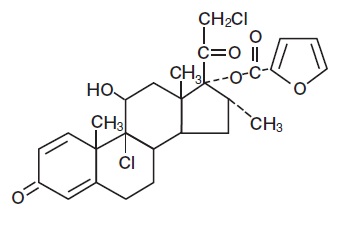
Chemically, mometasone furoate is 9α, 21-dichloro-11β,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17-(2-furoate), with the empirical formula C27H30Cl2O6, a molecular weight of 521.4 and the following structural formula:
Mometasone furoate is a white to off-white powder insoluble in water, freely soluble in acetone and in methylene chloride and sparingly soluble in heptane.
Each gram of Mometasone Furoate Topical Solution USP, 0.1% contains 1 mg mometasone furoate in a colorless, clear to translucent solution base of isopropyl alcohol (40%), hexylene glycol, hydroxypropyl cellulose, sodium phosphate monobasic anhydrous, purified water, glycerin and oleic acid. May also contain phosphoric acid used to adjust the pH to approximately 4.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Like other topical corticosteroids, mometasone furoate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
12.2 Pharmacodynamics
Studies performed with mometasone furoate lotion indicate that it is in the medium range of potency as compared with other topical corticosteroids.
In a study evaluating the effects of mometasone furoate lotion on the HPA axis, 15 mL were applied without occlusion twice daily (30 mL per day) for 7 days to 4 adult subjects with scalp and body psoriasis. At the end of treatment, the plasma cortisol levels for each of the 4 subjects remained within the normal range and changed little from baseline [seeWarnings and Precautions (5.1)].
Sixty-five pediatric subjects ages 6 to 23 months, with atopic dermatitis, were enrolled in an open-label, HPA axis safety trial. Mometasone furoate lotion was applied once daily for approximately 3 weeks over a mean body surface area of 40% (range 16%-90%). In approximately 29% of subjects who showed normal adrenal function by Cortrosyn test before starting treatment, adrenal suppression was observed at the end of treatment with mometasone furoate lotion. The criteria for suppression were: basal cortisol level of ≤5 mcg/dL, 30-minute post-stimulation level of ≤18 mcg/dL, or an increase of <7 mcg/dL. Follow-up testing 2 to 4 weeks after stopping treatment, available for 8 of the subjects, demonstrated suppressed HPA axis function in 1 subject, using these same criteria [seeUse in Specific Populations (8.4)].
12.3 Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Studies in humans indicate that approximately 0.7% of the applied dose of mometasone furoate ointment enters the circulation after 8 hours of contact on normal skin without occlusion. A similar minimal degree of absorption of the corticosteroid from the lotion formulation would be anticipated. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of mometasone furoate lotion. Long-term carcinogenicity studies of mometasone furoate were conducted by the inhalation route in rats and mice. In a 2-year carcinogenicity study in Sprague Dawley rats, mometasone furoate demonstrated no statistically significant increase of tumors at inhalation doses up to 67 mcg/kg (approximately 0.04 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis). In a 19-month carcinogenicity study in Swiss CD-1 mice, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 160 mcg/kg (approximately 0.05 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis).
Mometasone furoate increased chromosomal aberrations in an in vitro Chinese hamster ovary cell assay, but did not increase chromosomal aberrations in an in vitro Chinese hamster lung cell assay. Mometasone furoate was not mutagenic in the Ames test or mouse lymphoma assay, and was not clastogenic in an in vivo mouse micronucleus assay, a rat bone marrow chromosomal aberration assay, or a mouse male germ-cell chromosomal aberration assay. Mometasone furoate also did not induce unscheduled DNA synthesis in vivo in rat hepatocytes.
In reproductive studies in rats, impairment of fertility was not produced in male or female rats by subcutaneous doses up to 15 mcg/kg (approximately 0.01 times the estimated maximum clinical topical dose from mometasone furoate lotion on a mcg/m2 basis).
-
14 CLINICAL STUDIES
The safety and efficacy of mometasone furoate lotion for the treatment of corticosteroid-responsive dermatoses was demonstrated in two vehicle-controlled trials, one in scalp psoriasis and one in seborrheic dermatitis. A total of 405 subjects (age range: 12-95 years) received mometasone furoate lotion (205 subjects) or the vehicle lotion applied once daily for 21 days.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Mometasone Furoate Topical Solution USP, 0.1% is colorless, clear to translucent and supplied in 30-mL (27.5 gram) (NDC 45802-118-59) and 60-mL (55 gram) (NDC 45802-118-46) bottles; boxes of one.
Store Mometasone Furoate Topical Solution, 0.1% at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the following:
- •
- Use Mometasone Furoate Topical Solution 0.1% as directed by the physician. It is for external use only.
- •
- Avoid contact with the eyes.
- •
- Advise patients to report any visual symptoms to their healthcare providers.
- •
- Do not use Mometasone Furoate Topical Solution 0.1% on the face, underarms, or groin areas.
- •
- Do not use Mometasone Furoate Topical Solution 0.1% for any disorder other than that for which it was prescribed.
- •
- Do not bandage or otherwise cover or wrap the treated skin area so as to be occlusive, unless directed by the physician.
- •
- Report any signs of local adverse reactions to the physician.
- •
- Advise patients not to use Mometasone Furoate Topical Solution 0.1% in the treatment of diaper dermatitis. Do not apply Mometasone Furoate Topical Solution 0.1% in the diaper area, as diapers or plastic pants may constitute occlusive dressing.
- •
- Discontinue therapy when control is achieved. If no improvement is seen within 2 weeks, contact the physician.
- •
- Do not use other corticosteroid-containing products with Mometasone Furoate Topical Solution 0.1% without first consulting with the physician.
- SPL UNCLASSIFIED SECTION
-
Patient Information
Mometasone Furoate (moe-MET-a-sone fur-oate) Topical Solution USP, 0.1%
Important information: Mometasone Furoate Topical Solution, 0.1% is for use on skin only.
Do not use Mometasone Furoate Topical Solution, 0.1% in your eyes, mouth, or vagina.
What is Mometasone Furoate Topical Solution, 0.1%?
- •
- Mometasone Furoate Topical Solution, 0.1% is a prescription medicine used on the skin (topical) for the relief of redness, swelling, heat, pain (inflammation) and itching, caused by certain skin problems in people 12 years of age and older.
- •
- It is not known if Mometasone Furoate Topical Solution, 0.1% is safe and effective for use in children under 12 years of age.
- •
- Mometasone Furoate Topical Solution, 0.1% should not be used in children under 12 years of age.
Do not use Mometasone Furoate Topical Solution, 0.1% if you are allergic to mometasone furoate or any of the ingredients in Mometasone Furoate Topical Solution, 0.1%. See the end of this leaflet for a complete list of ingredients in Mometasone Furoate Topical Solution, 0.1%.
Before using Mometasone Furoate Topical Solution, 0.1%, tell your healthcare provider about all your medical conditions, including if you:
- •
- have a skin infection at the site to be treated. You may also need medicine to treat the skin infection.
- •
- are pregnant or plan to become pregnant. It is not known if Mometasone Furoate Topical Solution, 0.1% will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if mometasone furoate passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take other corticosteroid medicines by mouth or use other products on your skin or scalp that contain corticosteroids.
How should I use Mometasone Furoate Topical Solution, 0.1%?
- •
- Use Mometasone Furoate Topical Solution, 0.1% exactly as your healthcare provider tells you to use it.
- •
- Apply a few drops of Mometasone Furoate Topical Solution, 0.1% to the affected skin area 1 time each day and rub it in lightly until it disappears.
- •
- Use Mometasone Furoate Topical Solution, 0.1% until the affected skin area is improved. Tell your healthcare provider if the treated skin area does not get better after 2 weeks of treatment.
- •
- Do not bandage, cover, or wrap the treated skin area unless your healthcare provider tells you to.
- •
- Mometasone Furoate Topical Solution, 0.1% should not be used to treat diaper rash or redness. Avoid using Mometasone Furoate Topical Solution, 0.1% in the diaper area if wearing diapers or plastic pants.
- •
- Avoid using Mometasone Furoate Topical Solution, 0.1% on the face, groin, or underarms (armpits).
- •
- Wash your hands after applying Mometasone Furoate Topical Solution, 0.1%.
What are the possible side effects of Mometasone Furoate Topical Solution, 0.1%?
Mometasone Furoate Topical Solution, 0.1% may cause serious side effects, including:
- •
- Mometasone Furoate Topical Solution, 0.1% can pass through your skin. Too much Mometasone Furoate Topical Solution, 0.1% passing through your skin can cause your adrenal glands to stop working properly. Your healthcare provider may do blood tests to check for adrenal gland problems.
- •
- Vision problems. Topical corticosteroids may increase your chance of developing vision problems such as cataract and glaucoma. Tell your healthcare provider if you develop blurred vision or other vision problems during treatment with Mometasone Furoate Topical Solution, 0.1%.
- •
- Skin problems. Skin problems may happen during treatment with Mometasone Furoate Topical Solution, 0.1%, including allergic reactions (contact dermatitis) and skin infections at the treatment site. Stop using Mometasone Furoate Topical Solution, 0.1% and tell your healthcare provider if you develop any skin reactions such as pain, tenderness, swelling, or problems healing during treatment with Mometasone Furoate Topical Solution, 0.1%.
The most common side effects of Mometasone Furoate Topical Solution, 0.1% include burning, itching, and inflammation of the hair follicle (folliculitis).
These are not all the possible side effects of Mometasone Furoate Topical Solution, 0.1%.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Mometasone Furoate Topical Solution, 0.1%?
- •
- Store Mometasone Furoate Topical Solution, 0.1% at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep Mometasone Furoate Topical Solution, 0.1% and all medicines out of the reach of children.
General information about the safe and effective use of Mometasone Furoate Topical Solution, 0.1%.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Mometasone Furoate Topical Solution, 0.1% for a condition for which it was not prescribed. Do not give Mometasone Furoate Topical Solution, 0.1% to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Mometasone Furoate Topical Solution, 0.1% that is written for health professionals.
What are the ingredients in Mometasone Furoate Topical Solution, 0.1%?
Active ingredient: mometasone furoate
Inactive ingredients: isopropyl alcohol (40%), hexylene glycol, hydroxypropyl cellulose, sodium phosphate monobasic anhydrous, purified water, glycerin and oleic acid. May also contain phosphoric acid used to adjust the pH to approximately 4.5.
Manufactured By Padagis
Yeruham, Israel
Distributed By
Padagis®
Allegan, MI 49010 • www.padagis.com
Rev 09-22
5D800 RC J7
This Patient Information has been approved by the U.S. Food and Drug Administration.
-
Principal Display Panel - Carton
Rx Only
NDC 45802-118-46
Mometasone Furoate Topical Solution USP, 0.1%
For Dermatologic Use Only.
Not for Ophthalmic Use.
60 mL (55 g)
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
MOMETASONE FUROATE
mometasone furoate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOMETASONE FUROATE (UNII: 04201GDN4R) (MOMETASONE - UNII:8HR4QJ6DW8) MOMETASONE FUROATE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) OLEIC ACID (UNII: 2UMI9U37CP) PHOSPHORIC ACID (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-118-59 1 in 1 CARTON 02/02/2009 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:45802-118-46 1 in 1 CARTON 12/11/2008 2 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077180 12/11/2008 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)