Label: CVS HEALTH NATURAL DAILY FIBER SUGAR FREE NATURAL FIBER- psyllium husk powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-934-11 - Packager: CVS Pharmacy,Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 28, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient: (in each TEASPOON)
- Purpose
- Uses:
-
Warnings:
Choking:
Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting, or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Allergy alert:
This product may cause allergic reaction in people sensitive to inhaled or ingested psyllium.
Ask a doctor before use if you have
- ▪
- a sudden change in bowel habits persisting for 2 weeks
- ▪
- abdominal pain, nausea or vomiting
-
Directions:
Put one dose into an empty glass. Mix this product (child or adult dose) with at least 8 ounces (a full glass) of water or other fluid. Taking this product without enough liquid may cause choking. See choking warning. Stir briskly and drink promptly. If mixture thickens, add more liquid and stir.
Adults 12 years & older:
1 rounded TEASPOON in 8 oz of liquid at the first sign of irregularity. Can be taken up to 3 times daily. Generally produces effect in 12-72 hours.
6 – 11 yrs :
½ adult dose in 8 oz of liquid, up to 3 times daily
Under 6 yrs:
Consult a doctor
Bulk forming fibers like psyllium husk may affect how well other medicines work. If you are taking a prescription medicine by mouth, take this product at least 2 hours before or 2 hours after the prescribed medicine. As your body adjusts to increased fiber intake, you may experience changes in bowel habits or minor bloating.
-
Other information:
- •
- each teaspoon contains: potassium 35 mg; sodium 5 mg
- •
- PHENYLKETONURICS: CONTAINS PHENYLALANINE, 25 mg per teaspoon
- •
- store at room temperature tightly closed to protect from humidity
- •
- Keep out of reach of children
- •
- do not use if printed inner seal is broken or missing
- •
- contains a 100% natural, therapeutic fiber
- Inactive ingredients:
-
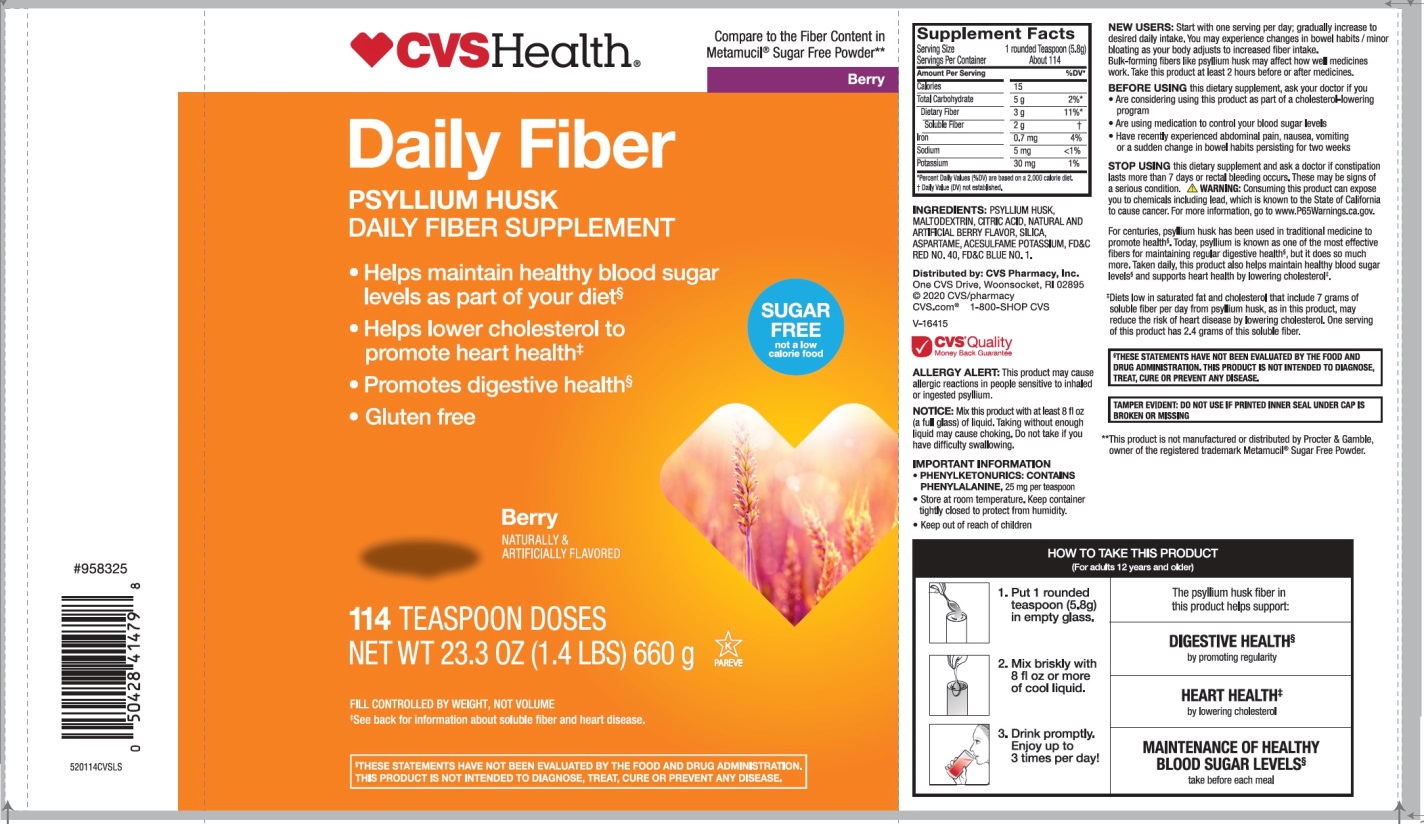
PRINCIPAL DISPLAY PANEL
CVS Health™
Compare to the active ingredient in Metamucil® 4 in 1 MultiHealth Fiber*
Berry
NDC 59779-934-11
Natural Daily Fiber
100% NATURAL PSYLLIUM HUSK
MULTI-BENEFIT DAILY FIBER SUPPLEMENT
THERAPY FOR REGULARITY
- •
- Helps you feel less hungry between meals**
- •
- Helps maintain healthy blood sugar levels as part of your diet**
- •
- Helps lower cholesterol to promote heart health†
- •
- Promotes digestive health**
- •
- Gluten free
SUGAR FREE not a low calorie food
Berry
NATURALLY & ARTIFICIALLY FLAVORED
114 TEASPOONS‡
NET WT 23.3 OZ (1.4 LB) (660g)
FILL CONTROLLED BY WEIGHT, NOT VOLUME
†See back for information about soluble fiber and heart disease.
‡Serving size varies. See DIRECTIONS on back panel for more information
GLUTEN FREE
If you have specific dietary needs, you should consult your doctor before consuming this product.
This product has a low glycemic index, a measure of the effect of dietary carbohydrates on blood sugar levels.
DO NOT USE IF PRINTED INNER SEAL IS BROKEN OR MISSING
**These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
*This product is not manufactured or distributed by Procter & Gamble, the distributor of Metamucil®.
4 in 1 MultiHealth Fiber*
Distributed by: CVS Pharmacy, Inc.
One CVS Drive,
Woonsocket, RI 02895
© 2020 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
CVS® Quality
Money Back Guarantee
-
INGREDIENTS AND APPEARANCE
CVS HEALTH NATURAL DAILY FIBER SUGAR FREE NATURAL FIBER
psyllium husk powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-934 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSYLLIUM HUSK (UNII: 0SHO53407G) (PSYLLIUM HUSK - UNII:0SHO53407G) PSYLLIUM HUSK 3.4 g in 5.78 g Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ASPARTAME (UNII: Z0H242BBR1) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-934-11 660 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/17/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 08/17/2015 Labeler - CVS Pharmacy,Inc. (062312574)