SODIUM CHLORIDE- sodium chloride injection
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
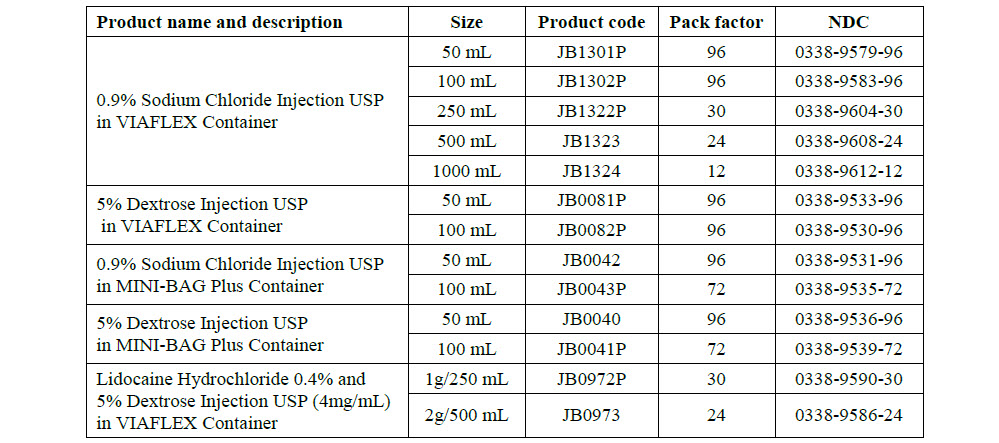
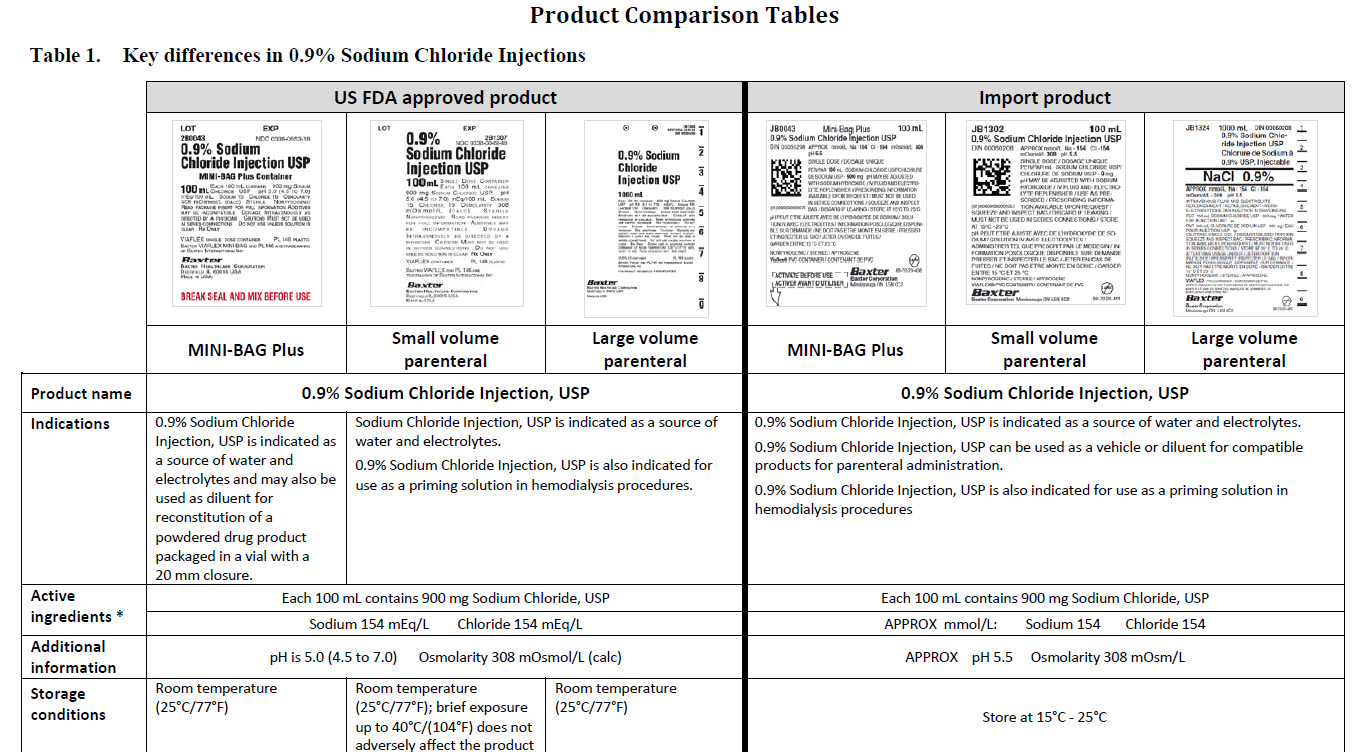
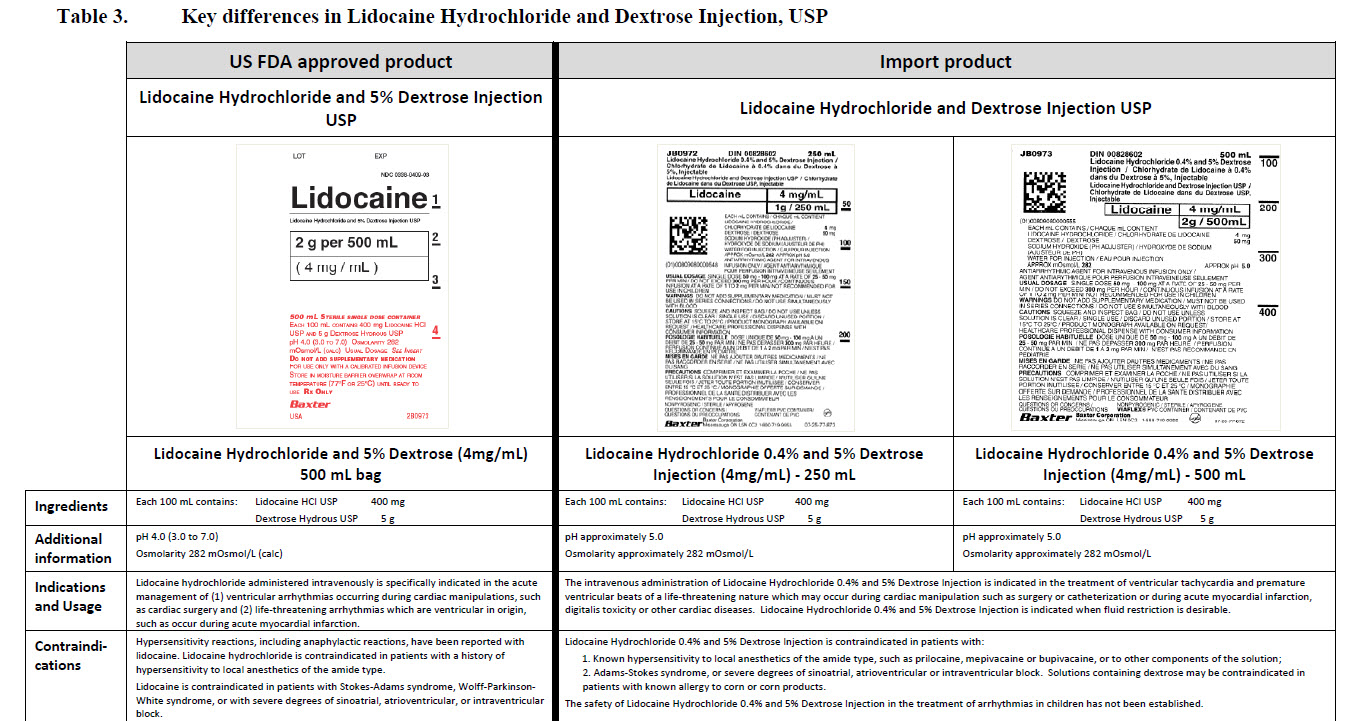
0.9% Sodium Chloride Injection, USP
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
JB1301
50 mL
0.9%Sodium Chloride Injection USP
DIN 00060208
APPROX mmol/L Na - 154 Cl - 154
mOsmol/L 308 pH 5.5
SINGLE DOSE/DOSAGE UNIQUE
Barcode (01)00809080001040
PER/PAR mL SODIUM CHLORIDE USP / CHLORURE DE SODIUM USP - 9 mg pH MAY BE ADJUSTED WITH SODIUM HYDROXIDE / IV FLUID AND ELECTROLYTE REPLENISHER USE AS PRESCRIBED / PRESCRIBING INFORMATION AVAILABLE UPON REQUEST / SQUEEZE AND INSPECT BAG / DISCARD IF LEAKING / MUST NOT BE USED IN SERIES CONNECTIONS / STORE AT 15°C TO 25°C
pH PEUT ETRE AJUSTE AVEC DE L’HYDROXYDE DE SODIUM SOLUTION / IV AVEC ELECTROLYTES ADMINISTRER TEL QUE PRESCRIT PAR LE MEDECIN / INFORMATION POSOLOGIQUE DISPONIBLE SUR DEMANDE / PRESSER ET INSPECTER LE SAC / JETER EN CAS DE FUITES / NE DOIT PAS ETRE MONTE EN SERIE / GARDER ENTRE 15 °C ET 25 °C
NONPYROGENIC / STERILE / APYROGENE
VIAFLEX® PVC CONTAINER / CONTENANT DE PVC
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No LATEX Logo
88-70-20-410
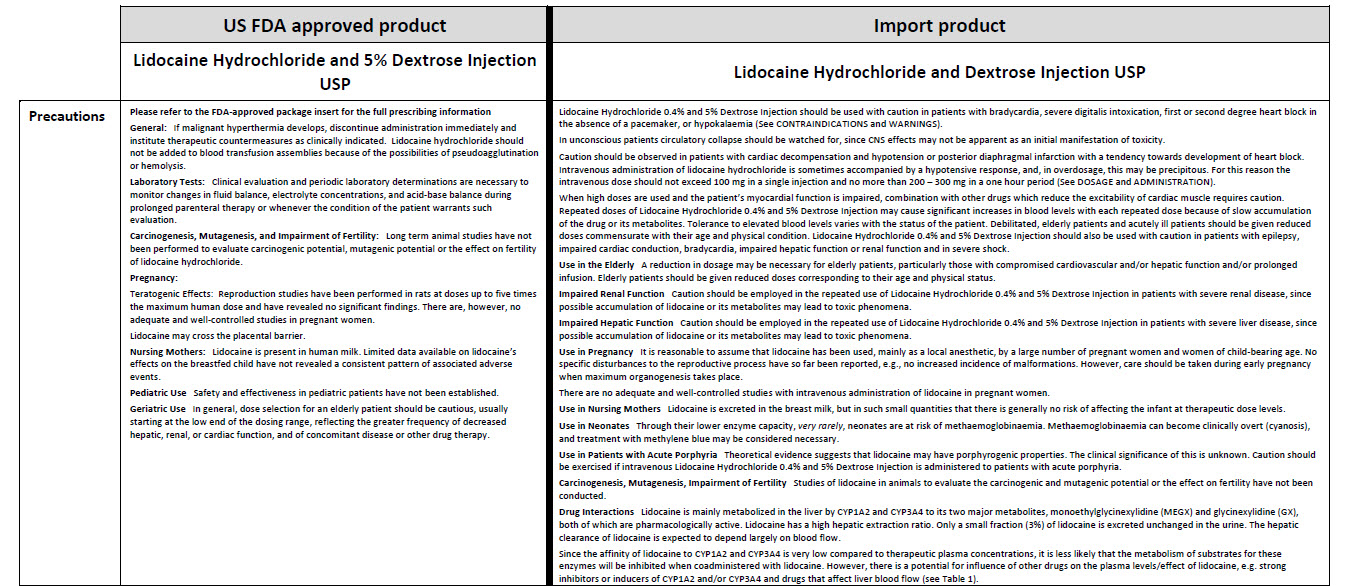
JB1302
100 mL
0.9% Sodium Chloride Injection USP
DIN 00060208
APPROX mmol/L Na - 154 Cl -154
mOsmol/L 308 pH 5.5
SINGLE DOSE / DOSAGE UNIQUE
Barcode (01)00809080001057
PER/PAR mL SODIUM CHLORIDE USP/
CHLORURE DE SODIUM USP - 9 mg
pH MAY BE ADJUSTED WITH SODIUM
HYDROXIDE / IV FLUID AND ELECTRO-
LYTE REPLENISHER / USE AS PRE-
SCRIBED / PRESCRIBING INFORMA-
TION AVAILABLE UPON REQUEST /
SQUEEZE AND INSPECT BAG / DISCARD IF LEAKING /
MUST NOT BE USED IN SERIES CONNECTIONS / STORE
AT 15°C - 25°C
pH PEUT ETRE AJUSTE AVEC DE L’HYDROXYDE DE SODIUM / SO-
LUTION IV AVEC ELECTROLYTES /
ADMINISTRER TEL QUE PRESCRIT PAR LE MEDECIN / IN-
FORMATION POSOLOGIQUE DISPONIBLE SUR DEMANDE
PRESSER ET INSPECTER LE SAC / JETER EN CAS DE
FUITES / NE DOIT PAS ETRE MONTE EN SERIE / GARDER
ENTRE 15 °C ET 25 °C
NONPYROGENIC / STERILE / APYROGENE
VIAFLEX® PVC CONTAINER / CONTENANT DE PVC
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No LATEX Logo
88-70-20-411
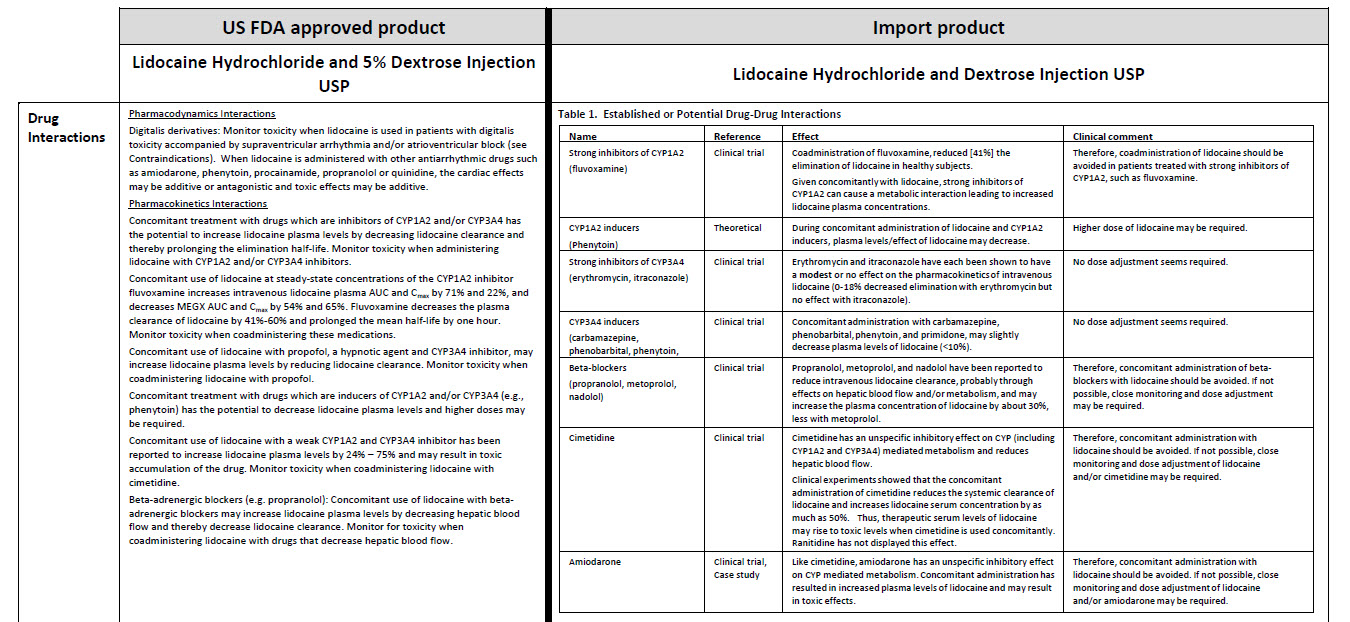
JB1322
250 mL
DIN 00060208
0.9% Sodium Chloride Injection USP
Chlorure de Sodium à 0.9% USP, Injectable
NaCl 0.9%
Barcode (01)00809080000562
APPROX mmol/L Na-154 Cl - 154
mOsmol/L 308
APPROX pH 5.5
INTRAVENOUS FLUID AND ELEC-
TROLYTE REPLENISHMENT / RET-
ABLISSEMENT HYDRO-ELECTRO-
LYTIQUE PAR INJECTION INTRA-
VEINEUSE
PER 100 mL SODIUM CHLORIDE USP - 900 mg / WATER
FOR INJECTION USP - qs / WATER FOR INJECTION USP
qs / pH MAY BE ADJUSTED WITH SODIUM HYDROXIDE
PAR 100 mL CHORURE DE SODIUM USP - 900 mg / EAU
POUR INJECTION USP - qs / pH PEUT ETRE AJUSTE
AVEC DE L’HYDROXYDE DE SODIUM
CAUTIONS SINGLE USE / DISCARD UNUSED PORTION /
SQUEEZE AND INSPECT BAG / PRESCRIBING INFORMA-
TION AVAILABLE UPON REQUEST / MUST NOT BE USED
IN SERIES CONNECTIONS / STORE AT 15°C TO 25°C
ATTENTIONS USAGE UNIQUE / JETER PORTION INUTILI-
SEE / PRESSER ET INSPECTER LE SAC / INFORMATION
POSOLOGIQUE DISPONIBLE SUR DEMANDE / NE DOIT
PAS ETRE MONTE EN SERIE / GARDER ENTRE 15°C ET
25°C
NONPYROGENIC / STERILE APYROGENE
VIAFLEX® PVC CONTAINER/CONTENANT DE PVC
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No LATEX Logo
88-70-20-415
50
100
150
200
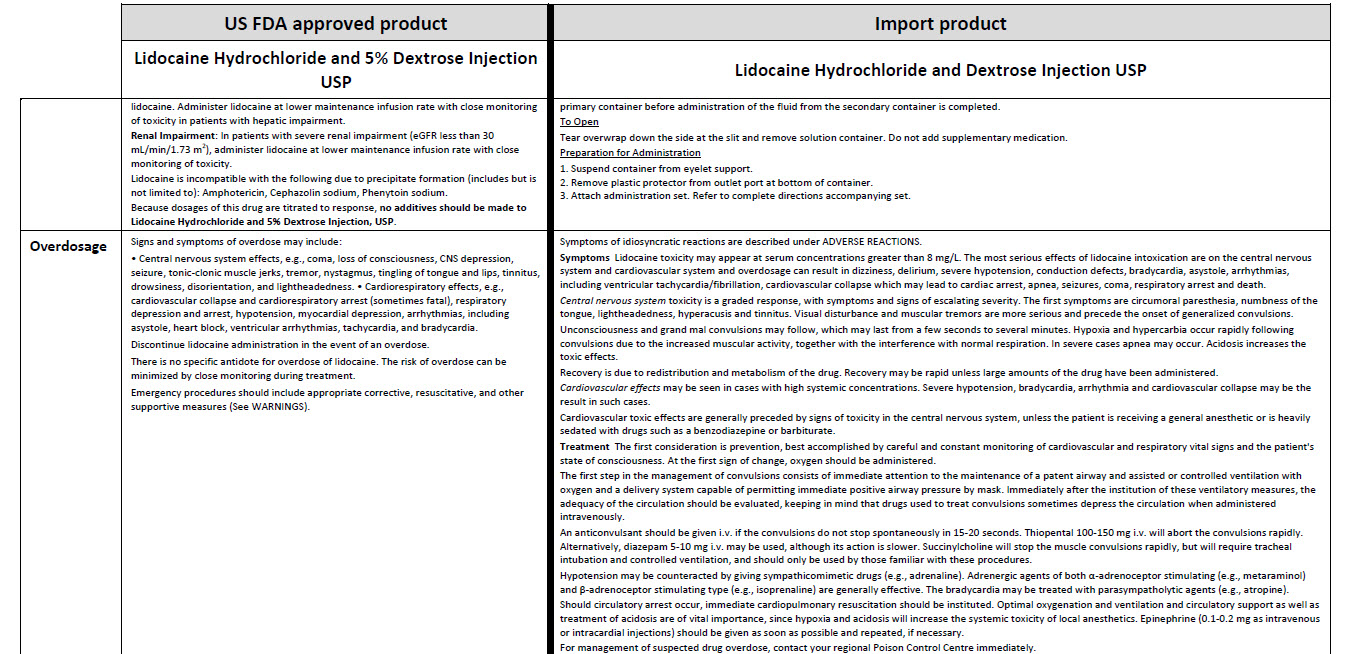
JB1323
500 mL
DIN 00060208
0.9% Sodium Chloride
Injection USP
Chlorure de Sodium à
0.9% USP, Injectable
NaCl 0.9%
APPROX mmol/L Na - 154 Cl - 154 mOsmol/L - 308 pH 5.5
RETABLISSEMENT HYDRO-ELECTROLYTIQUE / PAR INJECTION
INTRAVEINEUSE
PER 100 mL SODIUM CHLORIDE USP - 900 mg / WATER FOR
INJECTION USP – qs
PAR 100 mL CHLORURE DE SODIUM USP - 900 mg / EAU POUR
INJECTION USP - qs
CAUTIONS SINGLE USE / DISCARD UNUSED PORTION / SQUEEZE
AND INSPECT BAG / SEE DIRECTIONS FOR USE / MUST NOT BE
USED IN SERIES CONNECTIONS / STORE AT 15° C TO 25° C
ATTENTIONS USAGE UNIQUE / JETER PORTION INUTILISEE /
PRESSER ET INSPECTER LE SAC / VOIR MODE D’EMPLOI / NE DOIT
PAS ETRE MONTE EN SERIE / GARDER ENTRE 15° C ET 25° C
NONPYROGENIC / STERILE / APYROGENE
VIAFLEX PVC CONTAINER/ CONTENANT DE PVC
BAXTER AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET VIAFLEX SONT DES MARQUES DE COMMERCE DE BAXTER
INTERNATIONAL INC
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No LATEX Logo
07-25-77-317
1
2
3
4
JB1324
1000 mL
DIN 00060208
0.9% Sodium Chlo-
ride Injection USP
Chlorure de Sodium à
0.9% USP, Injectable
NaCl 0.9%
APPROX mmol/L Na - 154 Cl - 154
mOsmol/L - 308 pH 5.5
INTRAVENOUS FLUID AND ELECTROLYTE
REPLENISHMENT / RETABLISSEMENT HYDRO-
ELECTROLYTIQUE PAR INJECTION INTRAVEINEUSE
PER 100 mL SODIUM CHLORIDE USP - 900 mg / WATER
FOR INJECTION USP – qs
PAR 100 mL CHLORURE DE SODIUM USP - 900 mg / EAU
POUR INJECTION USP – qs
CAUTIONS SINGLE USE / DISCARD UNUSED PORTION
SQUEEZE AND INSPECT BAG / PRESCRIBING INFORMA-
TION AVAILABLE UPON REQUEST / MUST NOT BE USED
IN SERIES CONNECTIONS / STORE AT 15° C TO 25° C
ATTENTIONS USAGE UNIQUE / JETER PORTION
INUTILISEE / PRESSER ET INSPECTER LE SAC / INFOR-
MATION POSOLOGIQUE DISPONIBLE SUR DEMANDE /
NE DOIT PAS ETRE MONTE EN SERIE / GARDER ENTRE
15° C ET 25° C
NONPYROGENIC / STERILE / APYROGENE
VIAFLEX PVC CONTAINER / CONTENANT DE PVC
BAXTER AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET VIAFLEX SONT DES MARQUES DE COMMERCE DE
BAXTER INTERNATIONAL INC
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No LATEX Logo
88-70-20-490
1
2
3
4
5
6
7
8
9
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |