LUFYLLIN- dyphylline tablet
Meda Pharmaceuticals Inc.
----------
Lufyllin®
(dyphylline tablets, USP, 200 mg)
Lufyllin®-400
(dyphylline tablets, USP, 400 mg)
IN-0521-11 Rev. 1/09
Description
LUFYLLIN (dyphylline), a xanthine derivative, is a bronchodilator available for oral administration as tablets containing 200 mg and 400 mg of dyphylline. Other ingredients: magnesium stearate, microcrystalline cellulose.
Chemically, dyphylline is 7-(2,3-dihydroxypropyl)-theophylline, a white, extremely bitter, amorphous powder that is freely soluble in water and soluble in alcohol to the extent of 2 g/100 mL. Dyphylline forms a neutral solution that is stable in gastrointestinal fluids over a wide range of pH.
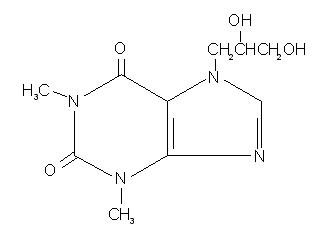
The molecular formula for dyphylline is C10H14N4O4 with a molecular weight of 254.25. Its structural formula is:
Clinical Pharmacology
Dyphylline is a xanthine derivative with pharmacologic actions similar to theophylline and other members of this class of drugs. Its primary action is that of bronchodilation, but it also exhibits peripheral vasodilatory and other smooth muscle relaxant activity to a lesser degree. The bronchodilatory action of dyphylline, as with other xanthines, is thought to be mediated through competitive inhibition of phosphodiesterase with a resulting increase in cyclic AMP producing relaxation of bronchial smooth muscle.
LUFYLLIN is well tolerated and produces less nausea than aminophylline and other alkaline theophylline compounds when administered orally. Unlike the hydrolyzable salts of theophylline, dyphylline is not converted to free theophylline in vivo. It is absorbed rapidly in therapeutically active form and in healthy volunteers reaches a mean peak plasma concentration of 17.1 mcg/mL in approximately 45 minutes following a single oral dose of 1000 mg of LUFYLLIN.
Dyphylline exerts its bronchodilatory effects directly and, unlike theophylline, is excreted unchanged by the kidneys without being metabolized by the liver. Because of this, dyphylline pharmacokinetics and plasma levels are not influenced by various factors that affect liver function and hepatic enzyme activity, such as smoking, age, congestive heart failure, or concomitant use of drugs which affect liver function.
The elimination half-life of dyphylline is approximately two hours (1.8-2.1 hr) and approximately 88% of a single oral dose can be recovered from the urine unchanged. The renal clearance would be correspondingly reduced in patients with impaired renal function. In anuric patients, the half-life may be increased 3 to 4 times normal.
Dyphylline plasma levels are dose-related and generally predictable. The range of plasma levels within which dyphylline can be expected to produce effective bronchodilation has not been determined.
Dyphylline plasma concentrations can be accurately determined using high pressure liquid chromatography (HPLC) or gas-liquid chromatography (GLC).
Indications and Usage
For relief of acute bronchial asthma and for reversible bronchospasm associated with chronic bronchitis and emphysema.
Warnings
LUFYLLIN is not indicated in the management of status asthmaticus, which is a serious medical emergency.
Although the relationship between plasma levels of dyphylline and appearance of toxicity is unknown, excessive doses may be expected to be associated with an increased risk of adverse effects.
Precautions
General: Use LUFYLLIN with caution in patients with severe cardiac disease, hypertension, hyperthyroidism, acute myocardial injury, or peptic ulcer.
Drug interactions: Synergism between xanthine bronchodilators (e.g., theophylline), ephedrine, and other sympathomimetic bronchodilators has been reported. This should be considered whenever these agents are prescribed concomitantly.
Concurrent administration of dyphylline and probenecid, which competes for tubular secretion, has been shown to increase the plasma half-life of dyphylline (see Clinical Pharmacology).
Carcinogenesis, mutagenesis, impairment of fertility: No long-term animal studies have been performed with LUFYLLIN.
Pregnancy: Teratogenic effects — Pregnancy Category C. Animal reproduction studies have not been conducted with LUFYLLIN. It is also not known if LUFYLLIN can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. LUFYLLIN should be given to a pregnant woman only if clearly needed.
Nursing mothers: Dyphylline is present in human milk at approximately twice the maternal plasma concentration. Caution should be exercised when LUFYLLIN is administered to a nursing woman.
Geriatric use: Clinical studies of LUFYLLIN did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Adverse Reactions
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-800-526-3840 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Adverse reactions with the use of LUFYLLIN have been infrequent, relatively mild, and rarely required reduction in dosage or withdrawal of therapy.
The following adverse reactions which have been reported with other xanthine bronchodilators, and which have most often been related to excessive drug plasma levels, should be considered as potential adverse effects when dyphylline is administered:
Gastrointestinal: nausea, vomiting, epigastric pain, hematemesis, diarrhea.
Central nervous system: headache, irritability, restlessness, insomnia, hyperexcitability, agitation, muscle twitching, generalized clonic and tonic convulsions.
Cardiovascular: palpitation, tachycardia, extrasystoles, flushing, hypotension, circulatory failure, ventricular arrhythmias.
Respiratory: tachypnea.
Renal: albuminuria, gross and microscopic hematuria, diuresis.
Other: hyperglycemia, inappropriate ADH syndrome.
Overdosage
There have been no reports, in the literature, of overdosage with LUFYLLIN. However, the following information based on reports of theophylline overdosage are considered typical of the xanthine class of drugs and should be kept in mind.
Signs and symptoms: Restlessness, anorexia, nausea, vomiting, diarrhea, insomnia, irritability, and headache. Marked overdosage with resulting severe toxicity has produced agitation, severe vomiting, dehydration, excessive thirst, tinnitus, cardiac arrhythmias, hyperthermia, diaphoresis, and generalized clonic and tonic convulsions. Cardiovascular collapse has also occurred, with some fatalities. Seizures have occurred in some cases associated with very high theophylline plasma concentrations, without any premonitory symptoms of toxicity.
Treatment: There is no specific antidote for overdosage with drugs of the xanthine class. Symptomatic treatment and general supportive measures should be instituted with careful monitoring and maintenance of vital signs, fluids, and electrolytes. The stomach should be emptied by inducing emesis if the patient is conscious and responsive, or by gastric lavage, taking care to protect against aspiration, especially in stuporous or comatose patients. Maintenance of an adequate airway is essential in case oxygen or assisted respiration is needed. Sympathomimetic agents should be avoided but sedatives such as short-acting barbiturates may be useful.
Dyphylline is dialyzable and, although not recommended as a routine procedure in overdosage cases, hemodialysis may be of some benefit when severe intoxication is present or when the patient has not responded to general supportive and symptomatic treatment.
Dosage and Administration
Dosage should be individually titrated according to the severity of the condition and the response of the patient.
Usual adult dosage: Up to 15 mg/kg every six hours.
Appropriate dosage adjustments should be made in patients with impaired renal function (see Clinical Pharmacology).
How Supplied
LUFYLLIN Tablets contain 200 mg dyphylline and are white, rectangular, scored on one side and imprinted WALLACE 521 on the other side. The tablets are available in bottles of 100 (NDC 0037-0521-92).
LUFYLLIN-400 Tablets contain 400 mg dyphylline and are white, capsule-shaped, scored on one side and imprinted WALLACE 731 on the other side. The tablets are available in bottles of 100 (NDC 0037-0731-92).
Storage: Store at controlled room temperature 20°-25°C (68°-77°F).
Dispense in a tight container.
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-800-526-3840 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Meda Pharmaceuticals
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
Printed in U.S.A. Rev. 1/09
IN-0521-11
Package Label - Principal Display Panel – 100-Count Bottle, 200 mg Tablets
NDC 0037-0521-92
100 Tablets
Lufyllin®
(dyphylline tablets, USP)
200 mg
Rx Only
Meda Pharmaceuticals
LB-052103-07 Rev. 8/07
Usual Dosage: See package insert.
Store at controlled room temperature 20°-25°C (68°-77°F)
Dispense in a tight container
Meda Pharmaceuticals
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120

Package Label - Principal Display Panel – 100-Count Bottle, 400 mg Tablets
NDC 0037-0731-92
100 Tablets
Lufyllin® - 400
(dyphylline tablets, USP)
400 mg
Rx Only
Meda Pharmaceuticals
LB-073103-07 Rev. 8/07
Usual Dosage: See package insert
Store at controlled room temperature 20°-25°C (68°-77°F)
Dispense in a tight container
Meda Pharmaceuticals
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120

| LUFYLLIN
dyphylline tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LUFYLLIN
dyphylline tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Meda Pharmaceuticals Inc. (051229602) |