Label: DOXYCYCLINE injection, powder, lyophilized, for solution
- NDC Code(s): 70771-1121-1, 70771-1121-6, 70771-1122-1
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
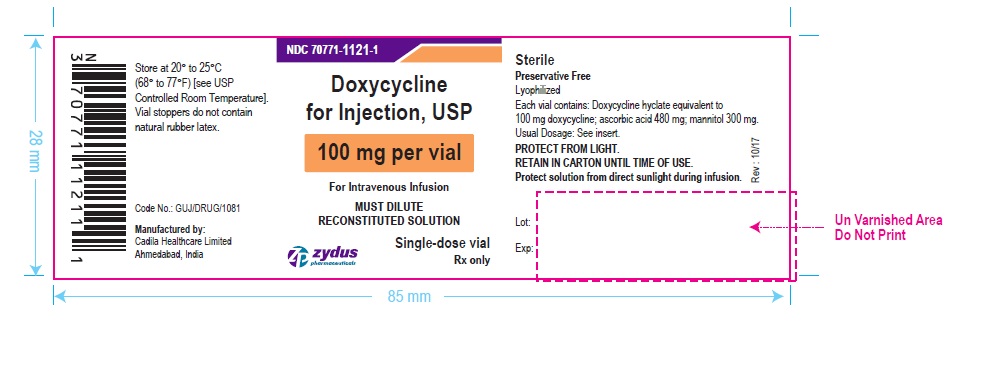
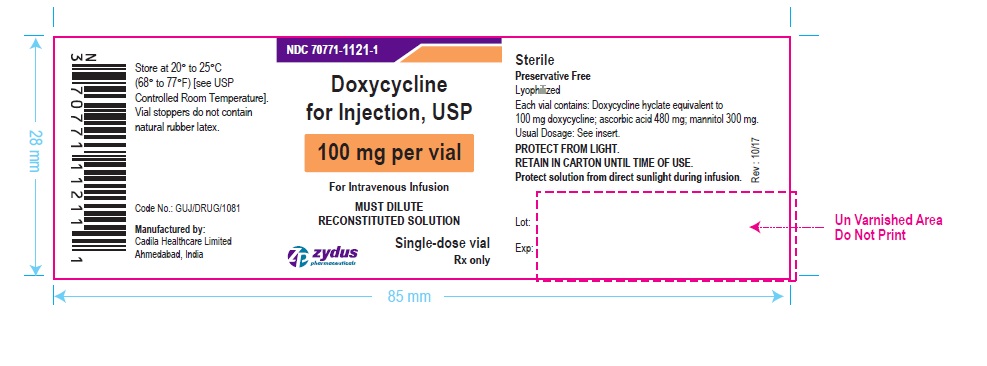
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL – DOXYCYCLINE 100 MG CONTAINER LABEL
NDC 70771-1121-1
Doxycycline for Injection, USP
100 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
Single-dose vial
Rx only
Zydus Pharmaceuticals
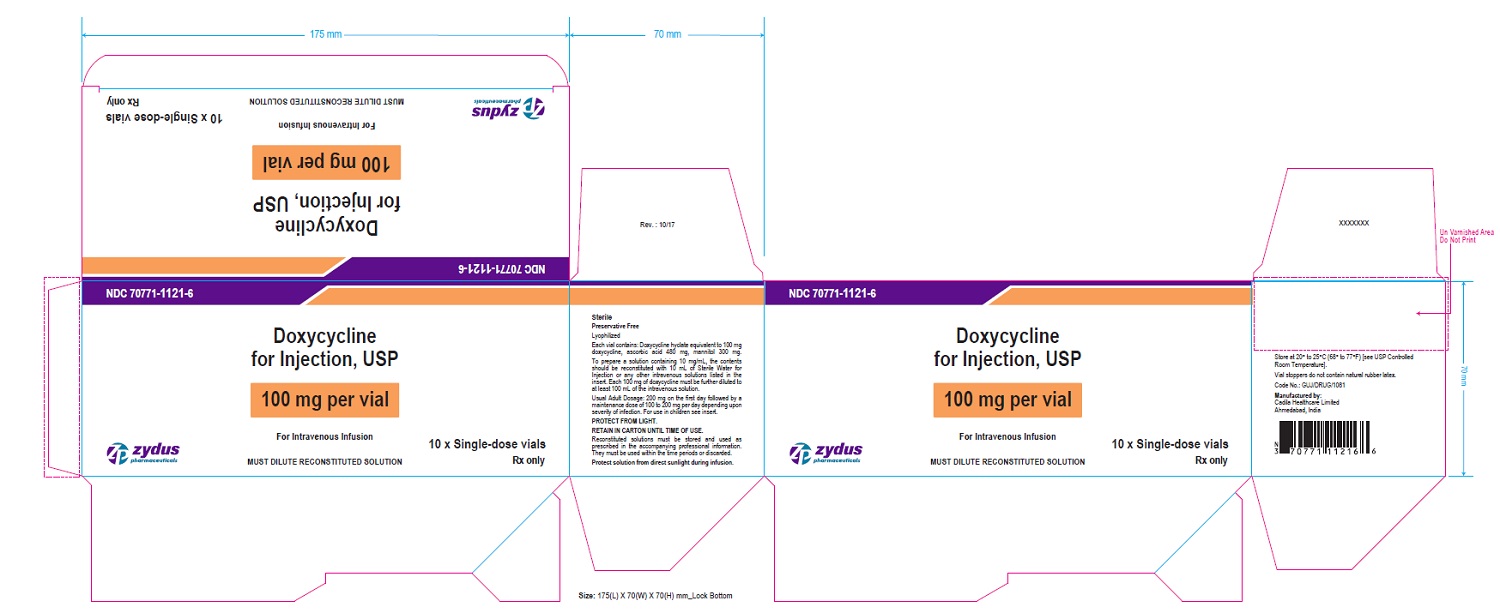
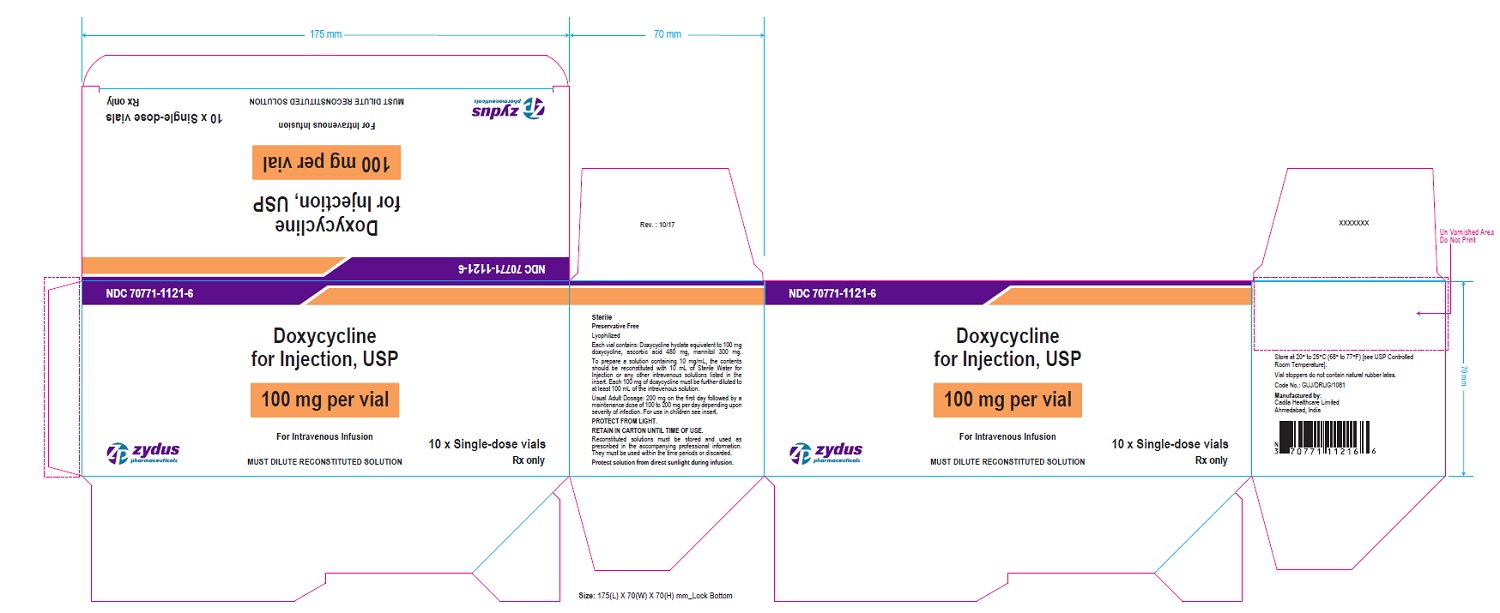
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - DOXYCYCLINE 100 MG CARTON LABEL
NDC 70771-1121-6
Doxycycline for Injection, USP
100 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
10 x Single-dose vials
Rx only
Zydus Pharmaceuticals
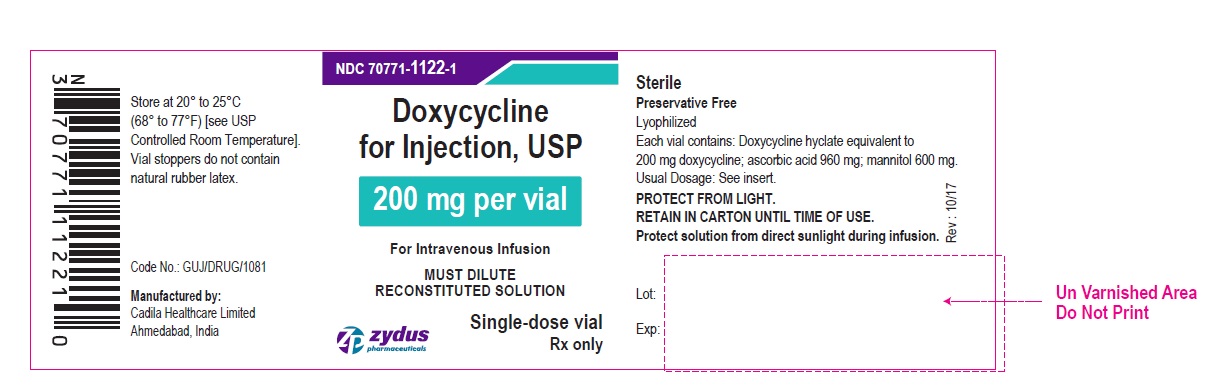
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL – DOXYCYCLINE 200 MG CONTAINER LABEL
NDC 70771-1122-1
Doxycycline for Injection, USP
200 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
Single-dose vial
Rx only
Zydus Pharmaceuticals
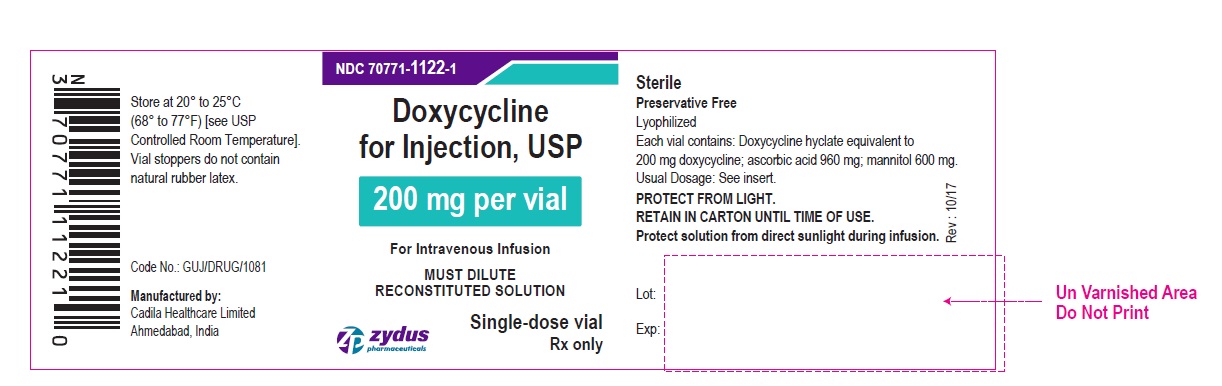
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - DOXYCYCLINE 200 MG CARTON LABEL
NDC 70771-1122-1
Doxycycline for Injection, USP
200 mg per vial
For Intravenous Infusion
MUST DILUTE RECONSTITUTED SOLUTION
Single-dose vial
Rx only
Zydus Pharmaceuticals

-
INGREDIENTS AND APPEARANCE
DOXYCYCLINE
doxycycline injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1121 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYCYCLINE HYCLATE (UNII: 19XTS3T51U) (DOXYCYCLINE ANHYDROUS - UNII:334895S862) DOXYCYCLINE ANHYDROUS 100 mg in 10 mL Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) 480 mg in 10 mL MANNITOL (UNII: 3OWL53L36A) 300 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1121-6 10 in 1 CARTON 02/01/2018 1 NDC:70771-1121-1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207757 02/01/2018 DOXYCYCLINE
doxycycline injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1122 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYCYCLINE HYCLATE (UNII: 19XTS3T51U) (DOXYCYCLINE ANHYDROUS - UNII:334895S862) DOXYCYCLINE ANHYDROUS 200 mg in 20 mL Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) 960 mg in 20 mL MANNITOL (UNII: 3OWL53L36A) 600 mg in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1122-1 1 in 1 CARTON 02/01/2018 1 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207757 02/01/2018 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1121, 70771-1122) , MANUFACTURE(70771-1121, 70771-1122) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 873671928 MANUFACTURE(70771-1121, 70771-1122) , ANALYSIS(70771-1121, 70771-1122)